Abstract
Summary
We studied 46,797 older adults who initiated denosumab in Ontario, Canada. Patient characteristics remained relatively stable over time and aligned with public reimbursement restrictions. Almost half of patients persisted with therapy for at least 3 years. Fifty-nine percent of patients who discontinued denosumab returned to treatment within 3.6 years.
Introduction
The purpose of this study was to describe the characteristics of patients who initiated denosumab and estimate persistence with therapy.
Methods
We identified older adults (aged ≥ 66 years) in Ontario who initiated denosumab between 2012/02 and 2015/03 and followed them to 2016/03. Patient characteristics were summarized using medical and pharmacy claims in the year before starting denosumab and osteoporosis drug use considered since 1996/10. Persistence with denosumab and return after discontinuation (> 90-day gap) were estimated using Kaplan–Meier curves. Analyses were stratified by community and long-term care (LTC) residence.
Results
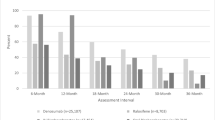
We identified 46,797 patients (monthly mean = 1263, SD = 187); 97% female, 13% LTC. Community-dwelling patients had a higher prevalence of bone mineral density testing (62% vs. 5%), yet were younger (mean age 78.5 vs. 86.6 years) and had lower prevalence of hip fractures (3% vs. 10%) compared to LTC patients. Eighty-two percent of patients had used osteoporosis medications in the past; 99% of whom took an oral bisphosphonate. Persistence was similar between community-dwelling and LTC patients: 59% persisted ≥ 2 years, 48% ≥ 3 years, and 38% ≥ 4 years, yet a larger proportion of LTC patients returned to denosumab after discontinuation (76% vs. 57%).
Conclusions
Denosumab utilization is increasing at a steady rate in Ontario. However, persistence remains a concern given the highly reversible pharmacokinetic profile of denosumab that results in a rapid increased fracture risk following discontinuation. Over 80% of patients had a history of oral bisphosphonate therapy, which may persist in bone despite discontinuing denosumab. Consequently, better understanding of denosumab safety and effectiveness among real-world users is important.


Similar content being viewed by others
References
Bekker PJ, Holloway DL, Rasmussen AS, Murphy R, Martin SW, Leese PT, Holmes GB, Dunstan CR, DePaoli AM (2004) A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res 19:1059–1066
Lewiecki EM, Miller PD, McClung MR, Cohen SB, Bolognese MA, Liu Y, Wang A, Siddhanti S, Fitzpatrick LA, AMG 162 Bone Loss Study Group (2007) Two-year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low BMD. J Bone Miner Res 22:1832–1841
Bone HG, Bolognese MA, Yuen CK, Kendler DL, Miller PD, Yang YC, Grazette L, San Martin J, Gallagher JC (2011) Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab 96:972–980
Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C, FREEDOM Trial (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765
Brown JP, Roux C, Torring O, Ho PR, Beck Jensen JE, Gilchrist N et al (2013) Discontinuation of denosumab and associated fracture incidence: analysis from the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial. J Bone Miner Res 28:746–752
McClung MR, Wagman RB, Miller PD, Wang A, Lewiecki EM (2017) Observations following discontinuation of long-term denosumab therapy. Osteoporos Int 28:1723–1732
Cummings SR, Ferrari S, Eastell R, Gilchrist N, Jensen JB, McClung M et al (2018) Vertebral fractures after discontinuation of Denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res 33:190–198
Prolia™ product monograph. Mississagua: Amgen Canada Inc.; 2010
Burden AM, Tadrous M, Calzavara A, Cadarette SM (2015) Uptake and characteristics of zoledronic acid and denosumab patients and physicians in Ontario, Canada: impact of drug formulary access. Osteoporos Int 26:1525–1533
Silverman SL, Siris E, Kendler DL, Belazi D, Brown JP, Gold DT, Lewiecki EM, Papaioannou A, Simonelli C, Ferreira I, Balasubramanian A, Dakin P, Ho P, Siddhanti S, Stolshek B, Recknor C (2015) Persistence at 12 months with denosumab in postmenopausal women with osteoporosis: interim results from a prospective observational study. Osteoporos Int 26:361–372
Papaioannou A, Khan A, Belanger A, Bensen W, Kendler D, Theoret F, Amin M, Brekke L, Erdmann M, Walker V, Adachi JD (2015) Persistence with denosumab therapy among osteoporotic women in the Canadian patient-support program. Curr Med Res Opin 31:1391–1401
Hadji P, Papaioannou N, Gielen E, Feudjo Tepie M, Zhang E, Frieling I, Geusens P, Makras P, Resch H, Möller G, Kalouche-Khalil L, Fahrleitner-Pammer A (2015) Persistence, adherence, and medication-taking behavior in women with postmenopausal osteoporosis receiving denosumab in routine practice in Germany, Austria, Greece, and Belgium: 12-month results from a European non-interventional study. Osteoporos Int 26:2479–2489
Fuksa L, Vytrisalova M (2015) Adherence to denosumab in the treatment of osteoporosis and its utilization in the Czech Republic. Curr Med Res Opin 31:1645–1653
Karlsson L, Lundkvist J, Psachoulia E, Intorcia M, Strom O (2015) Persistence with denosumab and persistence with oral bisphosphonates for the treatment of postmenopausal osteoporosis: a retrospective, observational study, and a meta-analysis. Osteoporos Int 26:2401–2411
Cheng L-I, Durden E, Limone B, Radbill L, Juneau PL, Spangler L, Mirza FM, Stolshek BS (2015) Persistance and compliance with osteroporosis therapies among women in a commercially insured population in the United States. J Manag Care Spec Pharm 21:824–833 833a
Lakatos P, Takacs I, Marton I, Toth E, Zoltan C, Lang Z et al (2016) A retrospective longitudinal database study of persistence and compliance with treatment of osteoporosis in Hungary. Calcif Tissue Int 98:215–225
Hadji P, Kyvernitakis I, Kann PH, Niedhart C, Hofbauer LC, Schwarz H, Kurth AA, Thomasius F, Schulte M, Intorcia M, Psachoulia E, Schmid T (2016) GRAND-4: the German retrospective analysis of long-term persistence in women with osteoporosis treated with bisphosphonates or denosumab. Osteoporos Int 27:2967–2978
Tremblay E, Perreault S, Dorais M (2016) Persistence with denosumab and zoledronic acid among older women: a population-based cohort study. Arch Osteoporos 11:30
Durden E, Pinto L, Lopez-Gonzalez L, Juneau P, Barron R (2017) Two-year persistence and compliance with osteoporosis therapies among postmenopausal women in a commercially insured population in the United States. Arch Osteoporos 12:22
Fahrleitner-Pammer A, Papaioannou N, Gielen E, Feudjo Tepie M, Toffis C, Frieling I et al (2017) Factors associated with high 24-month persistence with denosumab: results of a real-world, non-interventional study of women with postmenopausal osteoporosis in Germany, Austria, Greece, and Belgium. Arch Osteoporos 12:58
Reyes C, Tebe C, Martinez-Laguna D, Ali MS, Soria-Castro A, Carbonell C, Prieto-Alhambra D (2017) One and two-year persistence with different anti-osteoporosis medications: a retrospective cohort study. Osteoporos Int 28(10):2997–3004
Silverman SL, Siris E, Belazi D, Recknor C, Papaioannou A, Brown JP, Gold DT, Lewiecki EM, Quinn G, Balasubramanian A, Yue S, Stolshek B, Kendler DL (2018) Persistence at 24 months with denosumab among postmenopausal women with osteoporosis: results of a prospective cohort study. Arch Osteoporos 13:85
Cadarette SM, Burden AM (2010) Measuring and improving adherence to osteoporosis pharmacotherapy. Curr Opin Rheumatol 22:397–403
Levy AR, O'Brien BJ, Sellors C, Grootendorst P, Willison D (2003) Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol 10:67–71
Juurlink D PC, Croxford R, Chong A, Austin P, Tu J, Laupacis A (2006) Canadian Institute for Health Information Discharge Abstract Database: a validation study. ICES investigative report. Toronto: Institute for Clinical Evaluative Sciences
Cadarette SM, Jaglal SB, Raman-Wilms L, Beaton DE, Paterson JM (2011) Osteoporosis quality indicators using healthcare utilization data. Osteoporos Int 22:1335–1342
Xgeva™ product monograph. Mississauga, ON: Amgen Canada Inc.; 2016
David C, Confavreux CB, Mehsen N, Paccou J, Leboime A, Legrand E (2010) Severity of osteoporosis: what is the impact of co-morbidities? Joint Bone Spine 77(Suppl 2):S103–S106
Formulary search: Ontario drug benefit formulary/comparative drug index. Ontario Ministry of Health and Long-Term Care; 2017
SAS Enterprise Guide. 7.1 ed. Cary, NC: SAS Institute Inc.; 2014
Rothman KJ (2014) Six persistent research misconceptions. J Gen Intern Med 29:1060–1064
Greenland S, Senn SJ, Rothman KJ, Carlin JB, Poole C, Goodman SN, Altman DG (2016) Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol 31:337–350
Important changes to the availability and conditions of use for drugs containing calcitonin. Recalls and safety alerts. Ottawa: Health Canada; 2013
Paterson JM, Suleiman A, Hux JE, Bell C (2008) How complete are drug history profiles that are based on public drug benefit claims? Can J Clin Pharmacol 15:e108–e116
Ontario [Province] and Canada [Country]. Census Profile 2016 Census Statistics Canada Catalogue no 98-316-X2016001. Ottawa: Statistics Canada; 2017
Reams BD, O’Malley CD, Critchlow CW, Lauffenburger JC, Brookhart MA (2014) Changing patterns of use of osteoporosis medications in the years after launch: implications for comparative effectiveness research. Pharmacoepidemiol Drug Saf 23:251–260
Report on seniors falls in Canada. Ottawa: Public Health Agency of Canada, Minister of Public Works and Government Services; 2005
Hughes CM (2008) Compliance with medication in nursing homes for older people: resident enforcement or resident empowerment? Drugs Aging 25:445–454
Miller PD, Bolognese MA, Lewiecki EM, McClung MR, Ding B, Austin M, Liu Y, San Martin J, Amg Bone Loss Study Group (2008) Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone 43:222–229
Anastasilakis AD, Makras P (2016) Multiple clinical vertebral fractures following denosumab discontinuation. Osteoporos Int 27(5):1929–1930
Popp AW, Zysset PK, Lippuner K (2016) Rebound-associated vertebral fractures after discontinuation of denosumab-from clinic and biomechanics. Osteoporos Int 27:1917–1921
McClung MR (2016) Cancel the denosumab holiday. Osteoporos Int 27:1677–1682
Anagnostis P, Paschou SA, Mintziori G, Ceausu I, Depypere H, Lambrinoudaki I, Mueck A, Pérez-López FR, Rees M, Senturk LM, Simoncini T, Stevenson JC, Stute P, Trémollieres FA, Goulis DG (2017) Drug holidays from bisphosphonates and denosumab in postmenopausal osteoporosis: EMAS position statement. Maturitas 101:23–30
Russell RGG (2007) Determinants of structure-function relationships among bisphosphonates. Bone 40:S21–S25
Acknowledgements
We thank IMS Brogan Inc. for use of their Drug Information Database. This work was completed as part of Joann K. Ban’s M.Sc. thesis at the University of Toronto. Analyses were completed by Joann K. Ban at ICES University of Toronto, with support from the Leslie Dan Faculty of Pharmacy. Joann K. Ban held a training award stipend from the Canadian Institutes of Health Research Drug Safety and Effectiveness Cross-Disciplinary Training (DSECT) Program. Authors thank John-Michael Gamble for insightful discussions and M. Amine Amiche and Giulia P. Consiglio for analytical support.
Funding
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. Parts of this material are based on data and/or information compiled and provided by the Canadian Institutes of Health Information (CIHI). However, the analyses, conclusions, opinions, and statements expressed in the material are those of the authors and not necessarily those of CIHI.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ban, J., Hao, B., McCarthy, L. et al. Denosumab utilization among older adults in Ontario: patient characteristics, persistence with therapy, and return to therapy after an extended gap. Osteoporos Int 30, 1865–1872 (2019). https://doi.org/10.1007/s00198-019-05051-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-019-05051-1