Abstract
Summary
Treatment with zoledronic acid in osteoporotic patients with spinal fusion shortens the duration of time to fusion, improves the fusion rate, prevents the subsequent adjacent vertebral compression fractures, improves the clinical outcomes, and prevents immobilization-induced bone loss in the hip.
Introduction
The objective of the study was to explore the effects of zoledronic acid on the healing process in osteoporotic patients following spinal fusion in a randomized, placebo-controlled, and triple-blinded study.
Methods
Seventy-nine osteoporotic patients with single-level degenerative spondylolisthesis were randomly assigned to receive either zoledronic acid infusion (zoledronic acid group) or saline infusion (controls) after spinal fusion. Functional radiography and CT scans were used to evaluate fusion status. Bone formation was graded into three categories: Grade A (bridging bone bonding with adjacent vertebral bodies), Grade B (bridging bone bonding with either superior or inferior vertebral body), or Grade C (incomplete bony bridging). A solid fusion was defined as less than 5° of angular motion with Grade A or B bone formation. Adjacent vertebral compression fractures (VCF) were assessed on MRI at 12 months after surgery. Serum level of carboxy terminal cross-linked telopeptide of type I collagen (β-CTX) and amino-terminal propeptide of type I procollagen (PINP) was measured. Bone mineral density (BMD) was measured by DXA. Oswestry Disability Index (ODI) was used to assess the clinical outcomes.
Results
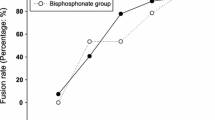
Grade A or B bridging bone was more frequently observed in zoledronic acid group at 3, 6, and 9 months post-operation compared to the control group (p < 0.05). At 12 -months post-operation, bridging bone and solid fusion were not significantly different between groups. No patients in zoledronic acid group showed aVCF, whereas six patients (17 %) in the control group did (p < 0.05). Both β-CTX and PINP were suppressed in zoledronic acid group. BMD at the femoral neck decreased rapidly and did not return to the preoperative level in the controls at 3 (−1.4 %), 6 (−2.5 %), and 12 (−0.8 %) months after surgery. Zoledronic acid prevented this immobilization-induced bone loss and increased BMD. ODI showed the improved clinical outcomes compared with controls at 9 and 12 months post-surgery.
Conclusion
Treatment with zoledronic acid in osteoporotic patients with spinal fusion shortens the time to fusion, improves the fusion rate, prevents subsequent aVCFs, and improves clinical outcomes.




Similar content being viewed by others
References
Etebar S, Cahill DW (1999) Risk factors for adjacent-segment failure following lumbar fixation with rigid instrumentation for degenerative instability. J Neurosurg 90(2 Suppl):163–169
Ponnusamy KE, Iyer S, Gupta G, Khanna AJ (2011) Instrumentation of the osteoporotic spine: biomechanical and clinical considerations. Spine J 11(1):54–63
Guppy K, Paxton L, Harris J, Alvarez J, Bernbeck J (2014) Does bone morphogenic protein (BMP) change the operative nonunion rates in spine fusions? Spine (Phila Pa 1976) 39(22):1831–1839
Manolagas SC, Jilka RL (1995) Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med 332(5):305–311
Tella SH, Gallagher JC (2014) Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol 142:155–170
Dai Z, Dai R, Xiao Y et al (2010) Progress in anti-osteoporosis drug treatment. Chin J Osteoporos 16(11):894–906
Schimmer RC, Bauss F (2003) Effect of daily and intermittent use of ibandronate on bone mass and bone turnover in postmenopausal osteoporosis: a review of three phase II studies. Clin Ther 25(1):19–34
Zou X, Xue Q, Li H, Bünger M, Lind M, Bünge C (2003) Effect of alendronate on bone ingrowth into porous tantalum and carbon fiber interbody devices: an experimental study on spinal fusion in pigs. Acta Orthop Scand 74(5):596–603
Xue Q, Li H, Zou X et al (2010) Alendronate treatment improves bone-pedicle screw interface fixation in posterior lateral spine fusion: an experimental study in a porcine model. Int Orthop 34(3):447–451
Huang RC, Khan SN, Sandhu HS et al (2005) Alendronate inhibits spine fusion in a rat model. Spine 30(22):2516–2522
Nagahama K, Kanayama M, Togawa D, Hashimoto T, Minami A (2011) Does alendronate disturb the healing process of posterior lumbar interbody fusion? A prospective randomized trial. J Neurosurg Spine 14(4):500–507
Recker RR, Delmas PD, Halse J et al (2008) Effects of intravenous zoledronic acid once yearly on bone remodeling and bone structure. J Bone Miner Res 23(1):6–16
Seeman E, Martin TJ (2015) Co-administration of antiresorptive and anabolic agents: a missed opportunity. J Bone Miner Res 30(5):753–764
Bransford R, Goergens E, Briody J et al (2007) Effect of zoledronic acid in an L6-L7 rabbit spine fusion model. Eur Spine J 16(4):557–562
Fairbank JC, Pynsent PB (2000) The oswestry disability index. Spine 25(22):2940–2952
Russell RG, Watts NB, Ebetino FH, Rogers MJ (2008) Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int 19(6):733–759
Gamsjaeger S, Buchinger B, Zwettler E et al (2011) Bone material properties in actively bone-forming trabeculae in postmenopausal women with osteoporosis after three years of treatment with once-yearly zoledronic acid. J Bone Miner Res 26(1):12–18
Bransford R, Goergens E, Briody J et al (2007) Effect of zoledronic acid in an L6-L7 rabbit spine fusion model. Eur Spine J 16(4):557–562
Yalçin N, Öztürk A, Ozkan Y (2011) The effects of zoledronic acid and hyperbaric oxygen on posterior lumbar fusion in a rabbit model. J Bone Joint Surg Br 93(6):793–800
Tu CW, Huang KF, Hsu HT et al (2014) Zoledronic acid infusion for lumbar interbody fusion in osteoporosis. J Surg Res 192(1):112–116
Boonen S, Orwoll E, Magaziner J et al (2011) Once-yearly zoledronic acid in older men compared with women with recent hip fracture. J Am Geriatr Soc 59(11):2084–2090
Tamma R, Zallone A (2012) Osteoblast and osteoclast crosstalks: from OAF to Ephrin. Inflamm Allergy Drug Targets 11(3):196–200
Ohtori S, Inoue G, Orita S et al (2013) Comparison of teriparatide and bisphosphonate treatment to reduce pedicle screw loosening after lumbar spinal fusion surgery in postmenopausal women with osteoporosis from a bone quality perspective. Spine 38(8):E487–492
Acknowledgments
This research was supported by “the Fundamental Research Funds for the Central Universities” (2012QNZT148), Science and Technology Plan of Hunan Province (2012WK3039) and National Institutes of Health (P01 CA093900)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The ethics committee of the Central South University approved the study.
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Chen, F., Dai, Z., Kang, Y. et al. Effects of zoledronic acid on bone fusion in osteoporotic patients after lumbar fusion. Osteoporos Int 27, 1469–1476 (2016). https://doi.org/10.1007/s00198-015-3398-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3398-1