Abstract
Summary
Dextromethorphan (DXM), a commonly used antitussive, is a dextrorotatory morphinan. Here, we report that DXM inhibits the receptor activator of nuclear factor kappa B ligand (RANKL)-induced osteoclastogenesis and bone resorption by abrogating the activation of NF-κB signalling in vitro. Oral administration of DXM ameliorates ovariectomy (OVX)-induced osteoporosis in vivo.
Introduction
DXM was reported to possess anti-inflammatory properties through inhibition of the release of pro-inflammatory factors. However, the potential role and action mechanism of DXM on osteoclasts and osteoblasts remain unclear. In this study, in vitro and in vivo studies were performed to investigate the potential effects of DXM on osteoclastogenesis and OVX-induced bone loss.
Methods
Osteoclastogenesis was examined by the TRAP staining, pit resorption, TNF-α release, and CCR2 and CALCR gene expression. Osteoblast differentiation was analyzed by calcium deposition. Osteogenic and adipogenic genes were measured by real-time PCR. Signaling pathways were explored using Western blot. ICR mice were used in an OVX-induced osteoporosis model. Tibiae were measured by µCT and serum markers were examined with ELISA kits.
Results
DXM inhibited RANKL-induced osteoclastogenesis. DXM mainly inhibited osteoclastogenesis via abrogation of IKK-IκBα-NF-κB pathways. However, a higher dosage of DXM antagonized the differentiation of osteoblasts via the inhibition of osteogenic signals and increase of adipogenic signals. Oral administration of DXM (20 mg/kg/day) partially reduced trabecular bone loss in ovariectomized mice.
Conclusion
DXM inhibits osteoclast differentiation and activity by affecting NF-κB signaling. Therefore, DXM at suitable doses may have new therapeutic applications for the treatment of diseases associated with excessive osteoclastic activity.






Similar content being viewed by others
References
Harada S, Rodan GA (2003) Control of osteoblast function and regulation of bone mass. Nature 423:349–355
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423:337–342
Lam J, Abu-Amer Y, Nelson CA, Fremont DH, Ross FP, Teitelbaum SL (2002) Tumour necrosis factor superfamily cytokines and the pathogenesis of inflammatory osteolysis. Ann Rheum Dis 61(Suppl 2):ii82–83
Romas E, Gillespie MT, Martin TJ (2002) Involvement of receptor activator of NFkappaB ligand and tumor necrosis factor-alpha in bone destruction in rheumatoid arthritis. Bone 30:340–346
Nakashima T, Wada T, Penninger JM (2003) RANKL and RANK as novel therapeutic targets for arthritis. Curr Opin Rheumatol 15:280–287
Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L (1997) A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390:175–179
Yasuda H, Shima N, Nakagawa N et al (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A 95:3597–3602
Araujo J, Logothetis C (2009) Targeting Src signaling in metastatic bone disease. Int J Cancer 124:1–6
Tortella FC, Pellicano M, Bowery NG (1989) Dextromethorphan and neuromodulation: old drug coughs up new activities. Trends Pharmacol Sci 10:501–507
Liu Y, Qin L, Li G, Zhang W, An L, Liu B, Hong JS (2003) Dextromethorphan protects dopaminergic neurons against inflammation-mediated degeneration through inhibition of microglial activation. J Pharmacol Exp Ther 305:212–218
Jiau SS, Cheng PY, Lee YM, Huang WH, Ko YF, Yen MH (2006) Beneficial effects of LK-4, an analog of dextromethorphan on lipopolysaccharide-induced sepsis in rats. J Biomed Sci 13:833–843
Liu SL, Li YH, Shi GY, Tang SH, Jiang SJ, Huang CW, Liu PY, Hong JS, Wu HL (2009) Dextromethorphan reduces oxidative stress and inhibits atherosclerosis and neointima formation in mice. Cardiovasc Res 82:161–169
Li G, Cui G, Tzeng NS, Wei SJ, Wang T, Block ML, Hong JS (2005) Femtomolar concentrations of dextromethorphan protect mesencephalic dopaminergic neurons from inflammatory damage. FASEB J 19:489–496
Lin TH, Tang CH, Hung SY, Liu SH, Lin YM, Fu WM, Yang RS (2010) Upregulation of heme oxygenase-1 inhibits the maturation and mineralization of osteoblasts. J Cell Physiol 222:757–768
Lin TH, Yang RS, Tang CH, Lin CP, Fu WM (2007) PPARgamma inhibits osteogenesis via the down-regulation of the expression of COX-2 and iNOS in rats. Bone 41:562–574
Lin TH, Tang CH, Wu K, Fong YC, Yang RS, Fu WM (2011) 15-Deoxy-delta(12,14) -prostaglandin-J2 and ciglitazone inhibit TNF-alpha-induced matrix metalloproteinase 13 production via the antagonism of NF-kappaB activation in human synovial fibroblasts. J Cell Physiol 226:3242–3250
Allan CM, Kalak R, Dunstan CR, McTavish KJ, Zhou H, Handelsman DJ, Seibel MJ (2010) Follicle-stimulating hormone increases bone mass in female mice. Proc Natl Acad Sci U S A 107:22629–22634
Teitelbaum SL (2000) Bone resorption by osteoclasts. Science 289:1504–1508
Sung B, Murakami A, Oyajobi BO, Aggarwal BB (2009) Zerumbone abolishes RANKL-induced NF-kappaB activation, inhibits osteoclastogenesis, and suppresses human breast cancer-induced bone loss in athymic nude mice. Cancer Res 69:1477–1484
DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M (1997) A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature 388:548–554
Ha H, Kwak HB, Lee SW, Jin HM, Kim HM, Kim HH, Lee ZH (2004) Reactive oxygen species mediate RANK signaling in osteoclasts. Exp Cell Res 301:119–127
Tang CH, Huang TH, Chang CS, Fu WM, Yang RS (2009) Water solution of onion crude powder inhibits RANKL-induced osteoclastogenesis through ERK, p38 and NF-kappaB pathways. Osteoporos Int 20:93–103
Mundy GR (2007) Osteoporosis and inflammation. Nutr Rev 65:S147–151
Hasturk H, Kantarci A, Ohira T, Arita M, Ebrahimi N, Chiang N, Petasis NA, Levy BD, Serhan CN, Van Dyke TE (2006) RvE1 protects from local inflammation and osteoclast- mediated bone destruction in periodontitis. FASEB J 20:401–403
Brennan FM, McInnes IB (2008) Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest 118:3537–3545
Sang CN (2000) NMDA-receptor antagonists in neuropathic pain: experimental methods to clinical trials. J Pain Symptom Manage 19:S21–25
Staud R, Vierck CJ, Robinson ME, Price DD (2005) Effects of the N-methyl-D-aspartate receptor antagonist dextromethorphan on temporal summation of pain are similar in fibromyalgia patients and normal control subjects. J Pain 6:323–332
Werling LL, Lauterbach EC, Calef U (2007) Dextromethorphan as a potential neuroprotective agent with unique mechanisms of action. Neurologist 13:272–293
Wang CC, Lee YM, Wei HP, Chu CC, Yen MH (2004) Dextromethorphan prevents circulatory failure in rats with endotoxemia. J Biomed Sci 11:739–747
Li G, Liu Y, Tzeng NS et al (2005) Protective effect of dextromethorphan against endotoxic shock in mice. Biochem Pharmacol 69:233–240
Chechneva OV, Mayrhofer F, Daugherty DJ, Pleasure DE, Hong JS, Deng W (2011) Low dose dextromethorphan attenuates moderate experimental autoimmune encephalomyelitis by inhibiting NOX2 and reducing peripheral immune cells infiltration in the spinal cord. Neurobiol Dis 44:63–72
Zou W, Amcheslavsky A, Takeshita S, Drissi H, Bar-Shavit Z (2005) TNF-alpha expression is transcriptionally regulated by RANK ligand. J Cell Physiol 202:371–378
Han KY, Yang D, Chang EJ, Lee Y, Huang H, Sung SH, Lee ZH, Kim YC, Kim HH (2007) Inhibition of osteoclast differentiation and bone resorption by sauchinone. Biochem Pharmacol 74:911–923
Consoli A, Devangelio E (2005) Thiazolidinediones and inflammation. Lupus 14:794–797
Okazaki R, Toriumi M, Fukumoto S, Miyamoto M, Fujita T, Tanaka K, Takeuchi Y (1999) Thiazolidinediones inhibit osteoclast-like cell formation and bone resorption in vitro. Endocrinology 140:5060–5065
Yang CR, Lai CC (2010) Thiazolidinediones inhibit TNF-alpha-mediated osteoclast differentiation of RAW264.7 macrophages and mouse bone marrow cells through downregulation of NFATc1. Shock 33:662–667
Kahn SE, Haffner SM, Heise MA et al (2006) Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355:2427–2443
Schwartz AV, Sellmeyer DE (2007) Thiazolidinediones: new evidence of bone loss. J Clin Endocrinol Metab 92:1232–1234
Karakawa A, Fukawa Y, Okazaki M, Takahashi K, Sano T, Amano H, Yamamoto M, Yamada S (2009) Diclofenac sodium inhibits NFkappaB transcription in osteoclasts. J Dent Res 88:1042–1047
Krischak GD, Augat P, Blakytny R, Claes L, Kinzl L, Beck A (2007) The non-steroidal anti-inflammatory drug diclofenac reduces appearance of osteoblasts in bone defect healing in rats. Arch Orthop Trauma Surg 127:453–458
Diaz-Rodriguez L, Garcia-Martinez O, Arroyo-Morales M, Rubio-Ruiz B, Ruiz C (2010) Effect of acetaminophen (paracetamol) on human osteosarcoma cell line MG63. Acta Pharmacol Sin 31:1495–1499
Canalis E, Mazziotti G, Giustina A, Bilezikian JP (2007) Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 18:1319–1328
Luppen CA, Leclerc N, Noh T, Barski A, Khokhar A, Boskey AL, Smith E, Frenkel B (2003) Brief bone morphogenetic protein 2 treatment of glucocorticoid-inhibited MC3T3-E1 osteoblasts rescues commitment-associated cell cycle and mineralization without alteration of Runx2. J Biol Chem 278:44995–45003
Capon DA, Bochner F, Kerry N, Mikus G, Danz C, Somogyi AA (1996) The influence of CYP2D6 polymorphism and quinidine on the disposition and antitussive effect of dextromethorphan in humans. Clin Pharmacol Ther 60:295–307
Reagan-Shaw S, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. FASEB J 22:659–661
Acknowledgement
The work was supported by a grant from Far Eastern Memorial Hospital—National Taiwan University Hospital Joint Research Program. We thank the NRPB (National Research Program for Biopharmaceuticals) Core Service Center (NSC 100-2312-B-002-007) for μCT analysis. We also thank Chung Gung University Animal Center and Taiwan Mouse Clinic for technical support in μCT experiments.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Karl Wu and Tzu-Hung Lin have equally contributed to this study
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Inhibition of high concentration of RANKL-induced NF-κB activation by dextromethorphan in RAW264.7 cells RAW264.7 cells were incubated with DXM (50 μM) for 30 min and then treated with high concentration of RANKL (100 ng/ml) for several time intervals. Western blot analysis showed that treatment with RANKL rapidly and markedly increased the phosphorylation of IKK and IκBα and enhanced IκBα degradation time dependently. Pretreatment with DXM decreased the activation of IKK and IκBα and inhibited the degradation of IκBα (n = 3) (TIFF 156 kb)
Fig. S2
Induction of adipogenic signals by dextromethorphan in osteoblasts cultured in adipogenic medium osteoblasts were treated with different concentrations of DXM in the presence of adipogenic medium for 3 days. mRNA was extracted by TriZol kit and was then analysed by RT-qPCR. a It was shown that administration of DXM higher than 1 μM significantly increased mRNA of LPL (b) and PPARgamma2 (c) (n = 4). Each value represents mean ± S.E.M. *, p < 0.05 as compared with control group without DXM (TIFF 872 kb)
Fig. S3
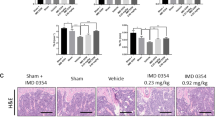
Effect of dextromethorphan on OVX-induced osteoclast activation. Bone resorption was determined by the serum level of c-terminal telopeptides of type-I collagen (CTX). The serum was collected from left ventricle 5 weeks after OVX surgery. DXM was put in drinking water and the dosage was evaluated by the quantity of water consumption. a DXM (20 mg/kg/day) significantly antagonized OVX-induced increase of serum level of osteoclastic marker. In addition, DXM also antagonized the increase of osteoclast number in proximal tibia. Representative TRAP staining images were shown in b and the quantitative data were shown in c. Scale bar: 100μm (n = 4–9). Each value represents mean ± S.E.M. *p < 0.05 as compared with Sham group. #p < 0.05 as compared with OVX alone group (TIFF 2,915 kb)
Fig. S4
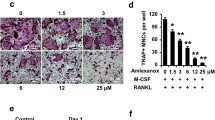
Dextromethorphan suppresses RANKL-induced TNF-α production in RAW264.7 cells. RAW264.7 cells were pretreated with different concentrations of DXM for 2 h before the addition of RANKL (10 ng/ml). The levels of TNF-α in the supernatant were measured by using TNF-α ELISA kit 16 h later. Each value represents the mean ± SEM of three independent experiments with triplicate cultures. *p < 0.05 as compared with RANKL-free control. #p < 0.05 as compared with RANKL-treatment alone group (TIFF 206 kb)
Rights and permissions
About this article
Cite this article
Wu, K., Lin, TH., Liou, HC. et al. Dextromethorphan inhibits osteoclast differentiation by suppressing RANKL-induced nuclear factor-κB activation. Osteoporos Int 24, 2201–2214 (2013). https://doi.org/10.1007/s00198-013-2279-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-013-2279-8