Abstract
Summary
The study goal was to compare simple two-dimensional (2D) analyses of bone strength using dual energy x-ray absorptiometry (DXA) data to more sophisticated three-dimensional (3D) finite element analyses using quantitative computed tomography (QCT) data. DXA- and QCT-derived femoral neck geometry, simple strength indices, and strength estimates were well correlated.
Introduction
Simple 2D analyses of bone strength can be done with DXA data and applied to large data sets. We compared 2D analyses to 3D finite element analyses (FEA) based on QCT data.
Methods
Two hundred thirteen women participating in the Study of Women's Health Across the Nation (SWAN) received hip DXA and QCT scans. DXA BMD and femoral neck diameter and axis length were used to estimate geometry for composite bending (BSI) and compressive strength (CSI) indices. These and comparable indices computed by Hip Structure Analysis (HSA) on the same DXA data were compared to indices using QCT geometry. Simple 2D engineering simulations of a fall impacting on the greater trochanter were generated using HSA and QCT femoral neck geometry; these estimates were benchmarked to a 3D FEA of fall impact.
Results
DXA-derived CSI and BSI computed from BMD and by HSA correlated well with each other (R = 0.92 and 0.70) and with QCT-derived indices (R = 0.83–0.85 and 0.65–0.72). The 2D strength estimate using HSA geometry correlated well with that from QCT (R = 0.76) and with the 3D FEA estimate (R = 0.56).
Conclusions
Femoral neck geometry computed by HSA from DXA data corresponds well enough to that from QCT for an analysis of load stress in the larger SWAN data set. Geometry derived from BMD data performed nearly as well. Proximal femur breaking strength estimated from 2D DXA data is not as well correlated with that derived by a 3D FEA using QCT data.


Similar content being viewed by others
References
Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A (2007) Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 22(3):465–475. doi:10.1359/jbmr.061113
Keaveny TM, Hoffmann PF, Singh M, Palermo L, Bilezikian JP, Greenspan SL, Black DM (2008) Femoral bone strength and its relation to cortical and trabecular changes after treatment with PTH, alendronate, and their combination as assessed by finite element analysis of quantitative CT scans. J Bone Miner Res 23(12):1974–1982. doi:10.1359/jbmr.080805
Keaveny TM, Kopperdahl DL, Melton LJ, Hoffmann PF, Amin S, Riggs BL, Khosla S (2010) Age-dependence of femoral strength in white women and men. J Bone Miner Res 25(5):994–1001. doi:10.1359/jbmr.091033
Beck TJ, Looker AC, Mourtada F, Daphtary MM, Ruff CB (2006) Age trends in femur stresses from a simulated fall on the hip among men and women: evidence of homeostatic adaptation underlying the decline in hip BMD. J Bone Miner Res 21(9):1425–1432. doi:10.1359/jbmr.060617
Karlamangla A, Barrett-Connor E, Young J, Greendale G (2004) Hip fracture risk assessment using composite indices of femoral neck strength: the Rancho Bernardo study. Osteoporos Int 15(1):62–70
Martin RB, Burr DB (1984) Non-invasive measurement of long bone cross-sectional moment of inertia by photon absorptiometry. J Biomech 17(3):195–201. doi:10.1016/0021-9290(84)90010-1
Camp JJ, Karwoski RA, Stacy MC, Atkinson EJ, Khosla S, Melton LJ, Riggs BL, Robb RA (2004) System for the analysis of whole-bone strength from helical CT images. In: Medical imaging 2004: physiology, function, and structure from medical images. SPIE, San Diego, pp 74–88
Keyak JH, Rossi SA, Jones KA, Skinner HB (1998) Prediction of femoral fracture load using automated finite element modeling. J Biomech 31(2):125–133. doi:S0021929097001231
Orwoll ES, Marshall LM, Nielson CM, Cummings SR, Lapidus J, Cauley JA, Ensrud K, Lane N, Hoffmann PR, Kopperdahl DL, Keaveny TM (2009) Finite element analysis of the proximal femur and hip fracture risk in older men. J Bone Miner Res 24(3):475–483. doi:10.1359/jbmr.081201
Sowers MR, Greendale GA, Bondarenko I, Finkelstein JS, Cauley JA, Neer RM, Ettinger B (2003) Endogenous hormones and bone turnover markers in pre- and perimenopausal women: SWAN. Osteoporos Int 14(3):191–197
Finkelstein JS, Lee M-LT, Sowers M, Ettinger B, Neer RM, Kelsey JL, Cauley JA, Huang M-H, Greendale GA (2002) Ethnic variation in bone density in premenopausal and early perimenopausal women: effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab 87(7):3057–3067. doi:10.1210/jc.87.7.3057
Beck TJ, Ruff CB, Warden KE, Scott WW, Gopala UR (1990) Predicting femoral neck strength from bone mineral data: a structural approach. Invest Radiol 25(1):6–18
Beck TJ, Looker AC, Ruff CB, Sievanen H, Wahner HW (2000) Structural trends in the aging femoral neck and proximal shaft: analysis of the third National Health and Nutrition Examination Survey dual-energy x-ray absorptiometry data. J Bone Miner Res 15(12):2297–2304. doi:10.1359/jbmr.2000.15.12.2297
Lewiecki EM, Keaveny TM, Kopperdahl DL, Genant HK, Engelke K, Fuerst T, Kivitz A, Davies RY, Fitzpatrick LA (2009) Once-monthly oral ibandronate improves biomechanical determinants of bone strength in women with postmenopausal osteoporosis. J Clin Endocrinol Metab 94(1):171–180. doi:10.1210/jc.2008-1807
Morgan EF, Keaveny TM (2001) Dependence of yield strain of human trabecular bone on anatomic site. J Biomech 34(5):569–577
Morgan EF, Bayraktar HH, Keaveny TM (2003) Trabecular bone modulus–density relationships depend on anatomic site. J Biomech 36(7):897–904
Bayraktar HH, Morgan EF, Niebur GL, Morris GE, Wong EK, Keaveny TM (2004) Comparison of the elastic and yield properties of human femoral trabecular and cortical bone tissue. J Biomech 37(1):27–35
Ramamurthi K, Ahmad O, Engelke K, Taylor R, Zhu K, Gustafsson S, Prince R, Wilson K (2011) An in vivo comparison of hip structure analysis (HSA) with measurements obtained by QCT. Osteoporos Int March 11:1–9. doi:10.1007/s00198-011-1578-1
Khoo BCC, Beck TJ, Qiao Q-H, Parakh P, Semanick L, Prince RL, Singer KP, Price RI (2005) In vivo short-term precision of hip structure analysis variables in comparison with bone mineral density using paired dual-energy X-ray absorptiometry scans from multi-center clinical trials. Bone 37(1):112–121. doi:10.1016/j.bone.2005.03.007
Verhulp E, van Rietbergen B, Huiskes R (2008) Load distribution in the healthy and osteoporotic human proximal femur during a fall to the side. Bone 42(1):30–35. doi:10.1016/j.bone.2007.08.039
Mayhew PM, Thomas CD, Clement JG, Loveridge N, Beck TJ, Bonfield W, Burgoyne CJ, Reeve J (2005) Relation between age, femoral neck cortical stability, and hip fracture risk. Lancet 366(9480):129–135. doi:10.1016/s0140-6736(05)66870-5
de Bakker PM, Manske SL, Ebacher V, Oxland TR, Cripton PA, Guy P (2009) During sideways falls proximal femur fractures initiate in the superolateral cortex: evidence from high-speed video of simulated fractures. J Biomech 42(12):1917–1925. doi:10.1016/j.jbiomech.2009.05.001
Schafer BW (2002) Local, Distortional, and Euler Buckling of Thin-Walled Columns. J Struct Eng 128(3):289–299
Lee T, Choi JB, Schafer BW, Segars WP, Eckstein F, Kuhn V, Beck TJ (2009) Assessing the susceptibility to local buckling at the femoral neck cortex to age-related bone loss. Ann Biomed Eng 37(9):1910–1920. doi:10.1007/s10439-009-9751-9
Treece GM, Gee AH, Mayhew PM, Poole KES (2010) High resolution cortical bone thickness measurement from clinical CT data. Med Image Anal 14(3):276–290. doi:10.1016/j.media.2010.01.003
McLeish R, Charnley J (1970) Abduction forces in the one-legged stance. J Biomech 3(2):191–209
Carter DR, Wilson CH (1976) Bone compressive strength: the influence of density and strain rate. Science 194(4270):1174–1176
Acknowledgments
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR), and the NIH Office of Research on Women's Health (ORWH) (grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495, AG026463). The bone strength and geometry data are from O.N. Diagnostics (CSI and BSI), Johns Hopkins University (HSA), and Mayo Clinic. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or the NIH.
Clinical centers: University of Michigan, Ann Arbor—Siobán Harlow, PI 2011, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA—Joel Finkelstein, PI 1999–present, Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL—Howard Kravitz, PI 2009–present, Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser—Ellen Gold, PI; University of California, Los Angeles—Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY—Carol Derby, PI 2011, Rachel Wildman, PI 2010–2011, Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry–New Jersey Medical School, Newark—Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, PA—Karen Matthews, PI.
NIH program office: National Institute on Aging, Bethesda, MD—Sherry Sherman 1994–present, Marcia Ory 1994–2001; National Institute of Nursing Research, Bethesda, MD—Program Officers.
Central laboratory: University of Michigan, Ann Arbor—Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating center: New England Research Institutes, Watertown, MA—Sonja McKinlay, Principal Investigator 1995–2001; University of Pittsburgh, Pittsburgh, PA–Kim Sutton-Tyrrell, Principal Investigator 2001–present.
Steering committee: Chris Gallagher, Chair; Susan Johnson, Chair
We thank the study staff at each site and all the women who participated in SWAN.
Conflicts of interest
Drs. Danielson, Atkinson, Cauley, Greendale, Greenspan, Karlamangla, Ruppert, and Vuga and Ms. Khaled and Ms. Lian have no conflicts of interest to report. Dr. Beck is co-founder of Beck Radiological Innovations, Inc., a company that develops software and hardware methods for measuring bone structure as well as other products. His former employer the Johns Hopkins University licensed the HSA software used in this paper to Hologic, Inc., and he receives a share of the royalties. Dr. Keaveny has a financial interest in O.N. Diagnostics, and both he and the company may benefit from the results of this work. Dr. Kopperdahl is employed by O.N. Diagnostics.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
2D beam analysis used for strength estimates by HSA and QCA methods
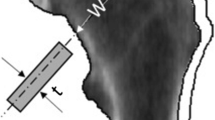
The free-body diagram of the femur simulating a fall impact is depicted in Fig. 1a. F I is the ground reaction force assumed to be equal to body weight (W), F H is the force acting through the center of the femoral head force assumed to be approximately equal to 5/6 of the body weight, and F S is femoral shaft force assumed to be equal to 1/6 of the body weight [26]. The shaft axis was assumed to be inclined 10° to the ground surface at impact (θ). Forces and moments were balanced to achieve static equilibrium, where l 1 and l 2 were derived as:
Internal forces in the neck resulting from external forces F I , F H, and F S are shown in Fig. 1b, where M F, P F, and V are bending moment, axial load, and shear force, respectively, on the cross section.
The small shear forces were neglected.
Using engineering beam theory, axial stresses on the medial and lateral surface of the femoral neck are computed:
where M F and P F are the bending moment and axial load on the cortical cross section, and y is the perpendicular distance from the neutral axis of bending (from HSA). By convention stresses on the lateral surface in compression are positive while at the medial surface, the bending moment produces negative tensile stress. Strength estimates were then derived as:
where σ y = ultimate yield stress in compression for cortical bone tissue [27]. Note that strength was also evaluated in tension on the medial surface in both HSA and QCA models, but without exception, values were smaller on the compressive surface, hence only the latter were used.
Rights and permissions
About this article
Cite this article
Danielson, M.E., Beck, T.J., Karlamangla, A.S. et al. A comparison of DXA and CT based methods for estimating the strength of the femoral neck in post-menopausal women. Osteoporos Int 24, 1379–1388 (2013). https://doi.org/10.1007/s00198-012-2066-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-012-2066-y