Abstract
Summary
In a cohort study of users of bisphosphonates, we evaluated the incidence of fragility fractures at all sites on the femur following for up to 8 years of therapy with alendronate or risedronate. We did not find evidence for a reversal of fracture protection with long-term use of bisphosphonates.
Introduction
Few studies have acquired adequate data with prolonged follow-up on bisphosphonate users in the general population to evaluate their long-term effects on the risk of hip fractures including those in the subtrochanteric region.
Methods
This cohort study utilizes a large USA database (January 1, 2000 to June 30, 2009). We compared patients with higher versus lower degrees of compliance [medication possession ratio, MPR <1/3 (the reference), 1/3–<2/3, or ≥2/3]. Radiographic adjudication of fracture site and features were not performed. Hazard ratios (HR) for fracture were estimated using time-dependent Cox models. Restricted cubic splines (RCS) were used to plot HRs for fracture against duration of therapy.
Results
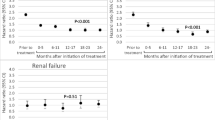
There were 3,655 incident cases of femoral fracture (764 subtrochanteric/shaft, 2,769 hip) identified during 917,741 person-years of follow-up (median = 3 years) on 287,099 patients (267,374 were women) from the date when they initiated oral bisphosphonate therapy. The corresponding HRs (95% confidence interval, CI) for overall femoral fractures associated with each additional year of therapy were 0.93 (0.86–1.01) within 5 years, and 0.89 (0.77–1.03) beyond 5 years for risedronate and 0.86 (0.81–0.91) and 0.95 (0.84–1.07) for alendronate, respectively. The corresponding estimates for subtrochanteric/shaft fractures were 1.05 (0.87–1.26) and 0.89 (0.60–1.33) for risedronate and 0.99 (0.92–1.05) and 1.05 (0.92–1.20) for alendronate, respectively. The HRs (95% CI) for overall femoral fractures associated with each additional year of alendronate or risedronate therapy within 5 and beyond 5 years were not significantly different.
Conclusion
Our study showed persistence of overall hip fracture protection with long-term use of alendronate or risedronate.


Similar content being viewed by others
References
Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. JAMA 2002;288:321
Pazianas M, Cooper C, Ebetino FH, Russell RG (2010) Long-term treatment with bisphosphonates and their safety in postmenopausal osteoporosis. Ther Clin Risk Manag 21(6):325–43
Wang Z, Bhattacharyya T (2011) Trends in incidence of subtrochanteric fragility fractures and bisphosphonate use among the US elderly, 1996–2007. J Bone Miner Res 26(3):553–60. doi:10.1002/jbmr.233
Park-Wyllie LY, Mamdani MM, Juurlink DN, Hawker GA, Gunraj N, Austin PC, Whelan DB, Weiler PJ, Laupacis A (2011) Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA 305(8):783–9
Pazianas M, Abrahamsen B (2011) Safety of bisphosphonates. Bone 49(1):103–10, Epub 2011 Jan 12. PubMed PMID: 21236370
Shane E, Burr D, Ebeling PR et al (2010) Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 25:2267–2294. doi:10.1002/jbmr.253
Rizzoli R, Akesson K, Bouxsein M, Kanis JA, Napoli N, Papapoulos S, Reginster JY, Cooper C (2011) Subtrochanteric fractures after long-term treatment with bisphosphonates: a European Society on Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, and International Osteoporosis Foundation Working Group Report. Osteoporos Int 22(2):373–90, Epub 2010 Nov 18
Adamson DM, Chang S, Hansen LG (2008) Health research data for the real world: the Marketscan databases. Thompson Healthcare, New York
Dobson AJ, Kuulasmaa K, Eberle E, Scherer J (1991) Confidence intervals for weighted sums of Poisson parameters. Stat Med 10(3):457–62
Fisher LD, Lin DY (1999) Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Public Health 20:145–57
Andrade SE, Kahler KH, Frech F, Chan KA (2006) Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf 15(8):565–74, discussion 575–7
Brankin E, Walker M, Lynch N, Aspray T, Lis Y, Cowell W (2006) The impact of dosing frequency on compliance and persistence with bisphosphonates among postmenopausal women in the UK: evidence from three databases. Curr Med Res Opin 22(7):1249–56
Curtis JR, Westfall AO, Cheng H, Lyles K, Saag KG, Delzell E (2008) Benefit of adherence with bisphosphonates depends on age and fracture type: results from an analysis of 101,038 new bisphosphonate users. J Bone Miner Res 23(9):1435–41
Heinzl H, Kaider A (1997) Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Comput Methods Programs Biomed 54(3):201–8
Durrleman S, Simon R (1989) Flexible regression models with cubic splines. Stat Med 8(5):551–61
Heinzl H, Kaider A, Zlabinger G (1996) Assessing interactions of binary time-dependent covariates with time in Cox proportional hazards regression models using cubic spline functions. Stat Med 15:25899–2601
Abrahamsen B, Eiken P, Eastell R (2010) Cumulative alendronate dose and the long-term absolute risk of subtrochanteric and diaphyseal femur fractures: a register-based national cohort analysis. J Clin Endocrinol Metab 95(12):5258–65, Epub 2010 Sep 15
Lefebvre G, Angers JF, Blais L (2006) Estimation of time-dependent rate ratios in case–control studies: comparison of two approaches for exposure assessment. Pharmacoepidemiol Drug Saf 15(5):304–16
Kim SY, Schneeweiss S, Katz JN, Levin R, Solomon DH (2011) Oral bisphosphonates and risk of subtrochanteric or diaphyseal femur fractures in a population-based cohort. J Bone Miner Res 26(5):993–1001. doi:10.1002/jbmr.288
Salminen ST, Pihlajamäki HK, Avikainen VJ, Böstman OM (2000) Population based epidemiologic and morphologic study of femoral shaft fractures. Clin Orthop Relat Res 372:241–9
Salminen ST. Femoral shaft fractures in adults: epidemiology, fracture patterns, nonunions, and fatigue fractures. Helsinki University Printing House, Helsinki 2005 ISBN 952-10-2523-9, http://www.doria.fi/bitstream/handle/10024/2183/femorals.pdf?sequence=1
Lenart BA, Neviaser AS, Lyman S, Chang CC, Edobor-Osula F, Steele B, van der Meulen MC, Lorich DG, Lane JM (2009) Association of low-energy femoral fractures with prolonged bisphosphonate use: a case control study. Osteoporos Int 20(8):1353–62, Epub 2008 Dec 9
Schilcher J, Michaëlsson K, Aspenberg P (2011) Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med 364(18):1728–37
Acknowledgments
The authors thank Jeff Agnew, YW, and Chad Melson who wrote the SAS codes and did the QC.
Conflicts of interest
MP: Consultancy, Warner Chilcott. BA: Grant/Research support from Novartis, Nycomed, Amgen, Merck Speakers Bureau with Nycomed, Merck, Eli Lilly. PE: Grant/Research support from Nycomed, Amgen, Speakers Bureau with Nycomed, Roche, Eli Lilly and Servier. RGGR: Research support from Procter and Gamble, Sanofi-Aventis, and Warner Chilcott, Consultant/speaker and legal activities Amgen, GlaxoSmithKline, Roche, Procter and Gamble, Sanofi-Aventis, Novartis, Eli Lilly, and Warner Chilcott.
Author information
Authors and Affiliations
Corresponding author
Additional information
Y. Wang is an ex-employee of P&G Pharmaceuticals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix 1
Baseline variables that were considered but did not make any appreciable difference in results included nutrient deficiency, diseases of the digestive system, genitourinary diseases, muskuloskeletal disorders, knee or hip surgeries, COPD, other respiratory diseases, disorders in the artery or vein systems, cerebral vascular disorders, other heart disease, pulmonary diseases, circulatory and ischemic heart disease separately, HBP, Parkinson’s, alcoholism, smoking, dementia, blood system disease, use of ACE inhibitors, anti-arrhythmic drugs, antibiotics, anticoagulants, ARBs, beta-blockers, BDZs, calcitonin, calcium channel blockers, anticonvulsants, digitalis, diuretics, hypoglycemics, NSAIDS, PPIs, psychotherapy, raloxifene, SSRIs, STATINS, and thyroxin. (DOC 319 kb)
Rights and permissions
About this article
Cite this article
Pazianas, M., Abrahamsen, B., Wang, Y. et al. Incidence of fractures of the femur, including subtrochanteric, up to 8 years since initiation of oral bisphosphonate therapy: a register-based cohort study using the US MarketScan claims databases. Osteoporos Int 23, 2873–2884 (2012). https://doi.org/10.1007/s00198-012-1952-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-012-1952-7