Abstract
Summary
Most post-vertebroplasty new-onset adjacent vertebral compression fractures (VCFs) occur within 2–3 months, and antiresorptive agents do not significantly reduce the risk of their occurrence. In opposite mechanism, teriparatide directly stimulates bone formation and improves bone strength and quality faster. The therapeutic effect of teriparatide is better than that of vertebroplasty combined with an antiresorptive treatment and is a potentially useful therapy for new-onset adjacent VCFs after vertebroplasty.
Introduction
Following vertebroplasty, patients are at increased risk of new-onset adjacent-level VCFs. The therapeutic effect of antiresorptive agents is too slow, and they are associated with the risk of new VCFs. Teriparatide markedly increases bone formation and strength and reduces the incidence of new-onset VCFs. This prospective cohort study compared the therapeutic effects of teriparatide with those of combined vertebroplasty and an anti-resorber for treating new-onset adjacent VCFs after vertebroplasty.
Methods
Fifty patients with adjacent VCFs were randomly assigned to two groups: teriparatide only (group A) and additional vertebroplasty combined with an antiresorptive agent (group B). Relevant clinical data of the two groups were prospectively compared.
Results
The 22 patients in group A were at higher risk of new VCFs than those in group B (22 patients); they were older and had more pre-existing fractures (p < 0.05). Patients treated with teriparatide had a significantly lower incidence of new-onset VCFs (odds ratio = 0.21; 95% confidence interval, 0.02–2.10). Teriparatide-mediated VCF reduction was 78.57%, which was markedly better than that of group B. The teriparatide group had a significant decrease in the visual analog scale and an increase in the Japanese Orthopedic Association low back pain score after 6 months of treatment (p < 0.05). The increase in lumbar spine BMD was marked in the teriparatide group (21.70% vs. 6.87%) after an 18-month treatment.
Conclusions
Treatment of post-vertebroplasty adjacent VCFs with teriparatide (no new vertebroplasty) was more effective than that of repeated vertebroplasties combined with an anti-resorber.
Similar content being viewed by others
Introduction
Vertebral compression fractures (VCFs) are the most common fragility fracture and are the hallmark of osteoporosis. Osteoporotic VCFs are associated with a significantly decreased quality of life and increased mortality in the elderly [1, 2]. Percutaneous vertebroplasty (PVP) has been performed for more than 10 years to treat painful osteoporotic VCFs. For patients with acute osteoporotic VCFs and persistent pain, PVP is effective and safe. Pain relief after vertebroplasty is immediate, sustained for at least 1 year, and is significantly greater than that achieved with conservative treatment, at an acceptable cost [3]. Nonetheless, clinical studies suggest patients who undergo vertebroplasty/kyphoplasty have a greater risk of new-onset VCFs in adjacent and non-adjacent spinal levels compared to patients with prior VCFs who did not undergo either procedure [4, 5]. Following vertebroplasty, patients are at an increased risk of new-onset adjacent-level VCFs, and when these fractures occur, they occur sooner than non-adjacent-level fractures [6]. Most new adjacent VCFs occurred within 3 months of PVPs [6, 7]. The relative risk of adjacent-level fracture was 4.62 times that of non-adjacent-level fracture. If treatment to prevent VCFs was not immediate and effective, new-onset VCFs occurred repeatedly within a few years after PVP [6–8]. Further fracture prevention is as important as pain relief in treating severe osteoporotic VCFs. Vertebroplasty does not play a role in further fracture prevention.
Antiresorptive agents are widely used to treat osteoporosis. Data from clinical trials show that antiresorptive agents (raloxifene and alendronate) reduce the risk of vertebral fracture by 40% to 50% after 3 years of treatment [9, 10]. Nonetheless, in the treatment of severely osteoporotic patients, the therapeutic effect of antiresorptive agents is too slow, and the use of these agents is associated with a high risk of new-onset fractures.
Teriparatide (rDNA origin) injection [recombinant human PTH(1–34)] is the first bone anabolic agent for the treatment of osteoporosis. Teriparatide administered once daily through subcutaneous self-injection results in a rapid and greater increase in vertebral bone mineral density (BMD) and a decreased risk of vertebral and non-vertebral fractures in postmenopausal women and men with osteoporosis [11, 12]. Teriparatide, with a mechanism different from that of antiresorptive agents, preferentially increases bone formation through direct early stimulation of osteoblasts. This increase in new bone formation results in a positive bone balance at the level of individual bone multicellular units and improved bone microarchitecture and quality [13]. We hypothesized that treating the adjacent VCF after PVP requires a faster increase in new bone formation and improved bone strength and quality. This prospective cohort study aimed to assess the immediate and mid-term efficacy and safety of teriparatide for treatment of new-onset adjacent compression fractures after PVP. We prospectively compared the therapeutic effects of teriparatide and combined vertebroplasty with an antiresorptive agent in fracture prevention, BMD increase, and sustained pain relief.
Patients and methods
Patients
All patients provided informed written consent before participating. We identified 50 patients who had adjacent VCFs after vertebroplasty from November 2007 to December 2010. VCF was diagnosed based on radiologic findings in all patients. All patients underwent magnetic resonance imaging (MRI) examinations for definitive diagnosis of new-onset osteoporotic VCFs when they had their first painful VCF. The exclusion criteria were spinal cancer, neurologic complications, osteoporotic vertebral collapse of greater than 90%, fracture through or destruction of the posterior wall, retropulsed bony fragmentation or bony fragments impinging on the spinal cord, medical conditions that would make the patient ineligible for emergency decompressive surgery if needed, and a likelihood of noncompliance with follow-up.
All subjects completed a baseline questionnaire that inquired about use of alcohol and cigarettes, rheumatic arthritis, history of spine or other bone fractures, and history of corticosteroid use. The patients were randomly assigned to either group A or group B (if the last number of their identification card was odd, they were assigned to group A (24 patients); if the last number was even, they were assigned to group B) (Fig. 1). Two patients in group A refused to accept daily subcutaneous injections of teriparatide and were excluded from this study. The remaining 22 patients in group A received subcutaneous injections of teriparatide (20 μg) once daily and daily supplementation with calcium (1,000–1,500 mg) and vitamin D (800–1,000 IU) throughout the study. These 22 patients were monitored for at least 20 months beginning with the diagnosis of post-PVP adjacent VCF (range, 20–36 months; mean, 25.05 ± 3.42 months).
Algorithm for the treatment of adjacent vertebral compression fractures. (*One patient in the teriparatide group experienced new-onset adjacent VCF. He did not receive vertebroplasty due to the VAS score less than 7 and the symptoms subsided after 2 weeks after continuing teriparatide treatment. **Four patients in the antiresorptive agents combined with vertebroplasty group received additional vertebroplasties.) VCF vertebral compression fracture, VP vertebroplasty, KP kyphoplasty, VAS visual analog scale, Loss loss of follow-up, Infarction large middle cerebral artery infarction
Twenty-six patients were assigned to group B, three were lost to follow-up, and one experienced a large middle cerebral artery infarction during the follow-up period. These four patients were excluded from the analysis. The remaining 22 patients in group B were given antiresorptive agents (alendronate or raloxifene) combined with calcium supplementation (1,000–1,500 mg) and vitamin D (800–1,000 IU) for osteoporosis treatment for at least 20 months after the occurrence of adjacent osteoporotic VCFs. The male patients were given alendronate treatment. For the female patients, if the last number of the medical record number was odd, raloxifene was used to treat the osteoporosis; if the last number was even, alendronate was used. The oral dosage of alendronate was 70 mg once weekly and that of raloxifene was 60 mg once daily. The antiresorptive agents were not combined. Patients who experienced side effects or had low compliance with their assigned antiresorptive agent were switched to the other agent. Two women had severe epigastric pain and nausea, and one woman had severe constipation after taking alendronate; these three patients were switched to raloxifene treatment. Two women had severe hot flashes, and one had intolerable leg cramps after taking raloxifene. These three women were switched to alendronate treatment. One of these antiresorptive agents had to be used for osteoporosis treatment for at least 18 months after an adjacent osteoporotic VCF occurred. If the patients in either group experienced new-onset VCFs, the painful vertebrae were located by a combination of local tenderness at the fracture site and the typical appearance of the fracture on radiographic (or MRI) evaluation. The indications for PVP were intense focal back pain, progressive kyphosis, and failure of conservative medical therapy in patients who had worn an orthosis for at least 1 month, if their visual analog scale (VAS) was less than 7. If the patient's VAS score was greater than 7 and conservative therapy for more than 2 weeks had failed, PVP was performed. The follow-up period for the 22 patients in group B was 24.63 ± 3.48 months (range, 20–36 months), beginning at the time post-PVP adjacent VCF was diagnosed.
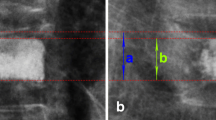
Clinical data on patients in both groups included age, sex, number of pre-existing VCFs, baseline BMD, bone mass index (BMI), the volume of polymethylmethacrylate (PMMA) injected during the first PVP, and the duration between new-onset VCFs (including adjacent and non-adjacent). For the vertebral reduction ratio (using a quantitative assessment) [14], we measured the anterior (Ha), posterior (Pa), adjacent posterior (Hpa), and middle (Hm) vertebral body height. In addition, the following ratios were calculated: anterior–posterior ratio = Ha/Hp, middle-posterior ratio = Hm/Hp, and posterior–posterior adjacent ratio = Hp/Hpa. The lowest value was defined as the vertebral reduction ratio.
Outcome assessment
Anteroposterior and lateral lumbar spine radiographs were obtained at baseline to determine whether at least two evaluable vertebrae in the lumbar spine region (L1–L4) were present in each patient fulfilling BMD entry criteria. Areal bone mineral density was measured in all patients by dual energy X-ray absorptiometry (DXA) using Hologic (Hologic Inc, Bedford, MA) or GE-lunar (Lunar Prodigy, GE Lunar Corp., Madison, USA) densitometers at baseline and at 6, 12, and 18 months after administration of teriparatide in group A and antiresorptive therapy in group B. Lumbar spine (L1–L4) measurements were obtained, and vertebrae with structural change or artifacts were excluded. Diagnoses were not made based on single vertebral bodies. The densitometries for each patient consistently used the same DXA system, acquisition methods, software, and young normal databases.
The Huskisson VAS [15] was used to estimate pain perception at baseline and at 1, 6, 12, and 18 months after administration of teriparatide. The standard scale from 0 (no pain) to 10 (intolerable pain) was used for pain analysis. The Japanese Orthopedic Association (JOA) low back pain scores [16] for clinical symptoms of patients with lower back pain were calculated at baseline and at 1, 6, 12, and 18 months. The JOA scores ranged from −6 to 29 points; the higher the score, the more normal is the patient's overall status. The JOA score is valuable for measuring improvement following treatment.
Statistical analysis
Results are presented as means ± SD. Independent data, including age, body mass index, pre-existing fracture, vertebral body reduction ratio, injected PMMA quantity, baseline BMD and T-score, and baseline VAS and JOA scores, were compared between groups A and B using the Mann–Whitney U test. The VAS scores and JOA scores at baseline and at 1, 6, 12, and 18 months after treatment were compared using Student's t test. A one-sample t test was also used to measure changes in BMD and T-score. Differences in sex ratios between the two groups were compared using the chi-square test. Statistical analyses were performed using SPSS (version 12.0; SPSS Inc, Chicago, IL). Unless otherwise stated, a p value <0.05 was taken as significant.
Results
Group A comprised 22 patients who received 18 months of teriparatide therapy for new-onset adjacent VCFs after PMMA vertebroplasty. The comparison group (group B) included 22 patients who received antiresorptive agents for at least 18 months. All 44 patients received vitamin D and calcium supplementation. Table 1 summarizes the comparison of clinical data between the two groups. There was no significant difference in male-to-female ratio, body mass index, injected volume of PMMA, steroid use, current smoking, alcohol drinking, or rheumatic arthritis between the two groups. The mean age of the patients in group A (75.59 ± 6.28) was significantly older than that of the patients in group B (70.55 ± 4.10, p = 0.002). The number of pre-existing VCFs was significantly higher in group A (3.01 ± 0.87) than in group B (2.17 ± 0.66, p = 0.004). The baseline BMD was 0.5796 ± 0.0816 g/cm2 in group A and 0.6245 ± 0.1026 g/cm2 in group B (p = 0.056). The vertebral body reduction ratio in group A was 48.68% ± 11.94%, while in group B, it was 49.82% ± 12.19% (p = 0.756).
Teriparatide (20 μg) was subcutaneously injected once daily, and oral calcium and vitamin D supplements were given for at least 18 months to the 22 patients in group A. Two patients experienced mild leg muscle spasms or cramps after injection of teriparatide. The symptoms subsided within 5 days in one patient and within 14 days in the other. The mean VAS score at baseline was 8.27 ± 1.16 (range, 6–10) (Fig. 2). After 1 month of treatment, the mean VAS score was 4.23 ± 0.97. The mean VAS score decreased to 2.23 ± 0.61 after 6 months, 1.20 ± 0.96 after 12 months, and 1.18 ± 0.80 (range, 0–3) after 18 months of teriparatide treatment (p = 0.001, all the differences between baseline and 6 months, 6 months and 12 months, and 12 months and 18 months were significant). Four patients had VAS scores of 0 and, six patients were analgesic free at the 18-month follow-up.
The mean VAS pain score and JOA lower back pain score changes in groups A and B. Data are expressed as mean ± SD. The decrease in VAS and the increase in JOA scores were significant between groups A and B at 6, 12, and 18 months, respectively. (*p < 0.05, ★p < 0.01) VAS visual analog scale, JOA Japanese Orthopedic Association
In group B, three patients had intolerable side effects and needed to change antiresorptive agents. The mean VAS score was 8.13 ± 0.95 (range, 6–10) prior to treatment and 4.09 ± 1.31 1 months after PVP plus antiresorptive agent treatment. The mean VAS score was 3.27 ± 1.42 after 6 months, 2.95 ± 1.56 after 12 months, and 3.14 ± 1.58 (range, 1–6) after 18 months of PVP plus antiresorptive treatment (Fig. 2). The VAS scores of all patients in group B were >0, and two patients were analgesic free at 18 months of follow-up. The VAS scores of the two groups were significantly different at each time point, beginning at 6 months (p < 0.05).
The mean JOA score in group A was 9.95 ± 4.02 prior to treatment and 18.59 ± 3.28 after 1 month of treatment. A significant increase in the mean JOA score occurred after 1 month of treatment with teriparatide. The mean JOA score was 21.23 ± 2.62 (range, 16–24; p = 0.001) after 6 months and 24.18 ± 2.79 after 12 months of teriparatide treatment and then increased to 26.00 ± 2.51 (range, 17–29) after 18 months of teriparatide treatment (p = 0.001, all the differences between baseline and 6 months, 6 months and 12 months, and 12 months and 18 months were significant). Three patients had full JOA scores, and four were analgesic free at 20 months of follow-up.
In group B, the mean JOA score was 11.59 ± 3.46 prior to treatment, 17.32 ± 3.41 after 1 month of treatment, 18.09 ± 2.58 (range, 16–24; p = 0.001) after 6 months of vertebroplasty combined with an antiresorptive treatment, and 19.41 ± 2.68 after 12 months of teriparatide treatment. After 18 months of treatment, the mean JOA score did not increase, but decreased slightly to 18.80 ± 3.33 (range, 13–26). No patient had a full JOA score, and two were analgesic free at 20 months of follow-up. The mean JOA scores of the two groups were significantly different at each time point, beginning at 6 months (p < 0.05).
The VAS score in group A was significantly lower than that in group B after 6 months of treatment (p = 0.003). Similarly, the JOA score in group A was significantly higher than in group B after 6 months (P = 0.000).
In group A (teriparatide group), only one patient developed a new-onset adjacent compression fracture after teriparatide treatment. That patient was a 72-year-old woman with severe osteoporosis (T-score, −4.30) who underwent vertebroplasty for an L2 compression fracture. A new-onset adjacent VCF at L3 occurred 78 days after PVP. The patient was started on teriparatide treatment on the day the new-onset fracture was diagnosed. Unfortunately, she experienced severe back pain again, and a plain lumbar spine X-ray film demonstrated a new L1 adjacent VCF after 27 days of teriparatide treatment. Teriparatide was continuously administrated. The intractable back pain subsided 2 weeks after teriparatide treatment. No additional VCF occurred, and the patient was symptom free at 33 months of follow-up. In group B, four (18.18%) patients developed new-onset VCFs (five vertebrae) after the second PVP, and received a third PVP. Among those four patients, one (25%) developed new-onset VCFs after the third PVP and required a fourth PVP (Fig. 3). The new-onset VCFs involved six vertebrae: four were adjacent VCFs (67%), and two were non-adjacent VCFs. Surgical complications resulting in the need for additional PVPs (six vertebrae) included one major bone cement extravasation requiring decompressive laminectomy, and two minor bone cement extravasations. Muscle power in both legs of the patient who underwent decompressive laminectomy recovered gradually but not fully during a 6-month rehabilitation program. Overall, teriparatide reduced the risk of new-onset VCFs after vertebroplasty (OR = 0.21; 95% CI, 0.02–2.10). The fracture risk reduction associated with teriparatide treatment was markedly better than that with antiresorptive agents; the relative risk reduction was 78.57%.
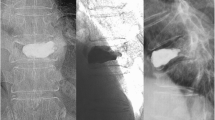
The patient was a 74-year-old woman with severe osteoporosis (T-score, −3.70) who underwent PVP for T10 and T12 VCFs (a). A new-onset adjacent VCF at T9 occurred 68 days after PVP, and the patient underwent a second PVP (b). The patient began alendronate treatment on the day the new-onset fracture was diagnosed. She experienced severe back pain again, and a plain lumbar spine X-ray demonstrated new L1 and L2 adjacent VCFs 43 days after the second PVP, so she underwent a third PVP due to intractable back pain (c). Unfortunately, a new T11 adjacent VCF occurred 33 days later, and a fourth PVP was performed 2 weeks later, after which minor bone cement extravasation occurred (d)
The BMD and T-score of the lumbar spine were estimated prior to and after 6, 12, and 18 months of treatment. All 22 patients in group A underwent BMD examination before administration of teriparatide. One patient had an L2 VCF and an adjacent L3 VCF before treatment with teriparatide and experienced a new adjacent L1 VCF 27 days after starting therapy. Therefore, only the L4 vertebral body was not involved, so the BMD data were not included. One of the remaining 21 patients missed the 6-month BMD examination, and another missed the 12-month BMD examination. Three patients in group B had only one evaluable vertebral body for DXA examination, so the BMD data were excluded. Five patients skipped the 6-month, two skipped the 12-month, and two skipped the 18-month DXA examination. The remaining 19 patients had at least two evaluable BMD data for analysis.
In group A, the mean BMD was 0.5796 ± 0.0816 g/cm2 at baseline, 0.6548 ± 0.1073 g/cm2 after 6 months, 0.6840 ± 0.1161 g/cm2 after 12 months, and 0.7054 ± 0.1030 g/cm2 after 18 months teriparatide treatment (Fig. 4), at which time, spinal BMD had increased 21.7%. The BMDs and T-scores increased markedly by the end of 6 months of therapy and increased slowly and steadily from the 6th month to the 18th month of treatment. The mean T-score value was −3.76 ± 0.71 at baseline, −3.16 ± 0.60 after 6 months, −3.00 ± 0.59 after 12 months, and −2.86 ± 0.53 after 18 months of teriparatide treatment (p = 0.000, all the differences between baseline and 6 months, 6 and 12 months, and 12 and 18 months were significant).
The mean lumbar spine BMD before and at 6, 12, and 18 months after treatment. Data are expressed as mean ± SD. The BMD increased markedly in group A by the end of 6 months of therapy, and continued to increase slowly and steadily from the 6th to the 18th month of treatment. The increase in lumbar spine BMD was marked in the teriparatide group (21.7% vs. 6.87%) after 18 months of treatment. (*p < 0.05, ★ p < 0.01) BMD bone mineral density
In group B, the mean BMD was 0.6245 ± 0.1026 g/cm2 at baseline, 0.6281 ± 0.0964 g/cm2 after 6 months, 0.6582 ± 0.1027 g/cm2 after 12 months, and 0.6705 ± 0.0894 g/cm2 after 18 months of antiresorptive treatment, at which time, spinal BMD had increased 6.87%. The mean T-score values were −3.43 ± 0.73 at baseline, −3.36 ± 0.64 after 6 months, −3.15 ± 0.63 after 12 months, and −3.12 ± 0.57 after 18 months of treatment with antiresorptive agents (p = 0.066).
Discussion
Vertebral fractures are the most common fragility fracture in osteoporotic patients and are associated with a 16% reduction in expected 5-year survival. Studies show that VCFs are often not diagnosed, and only about 30% of VCFs come to medical attention [17]. Vertebroplasty and kyphoplasty are minimally invasive procedures for the treatment of VCFs and are used primarily for pain relief and restoration of vertebral body height. Nonetheless, recent studies have questioned the effects of vertebroplasty [18, 19]. Buchbinder et al. found vertebroplasty had no beneficial effect compared with a sham procedure in patients with painful osteoporotic VCFs at 1 week and at 1, 3, or 6 months after treatment. They demonstrated vertebroplasty did not result in a significant advantage in any measured outcome at any time point [18]. Kallmes et al. demonstrated in a randomized controlled trial that improvements in pain and pain-related disability associated with osteoporotic VCFs in patients treated with vertebroplasty were similar to the improvements in a simulated procedure without the use of cement (control group) [20]. PVP appeared to relieve pain effectively and restore vertebral body height in most studies [3, 21]. Although PVP relieves the pain of compression fractures, recurrent back pain after PVP is common [21]. Among our group B patients, the VAS score was 2.95 ± 1.56 at month 12 and 3.14 ± 1.58 at month 18 (p = 0.329). The lack of statistical significance between these two periods shows that pain relief was not sustained. Teriparatide reduced fracture risk, and in a published meta-analysis of clinical trials, teriparatide-treated patients had a reduced incidence of back pain relative to a placebo and antiresorptive drugs [22, 23]. Patients randomized to teriparatide had a reduced risk of new or worsening back pain compared with patients randomized to a placebo, hormone replacement therapy, or alendronate [23]. Patients with osteoporosis treated with antiresorptive and anabolic agents, particularly those with teriparatide therapy, had a reduced risk of new or worsening back pain. Fewer patients treated with teriparatide reported new or worsening back pain, especially moderate and severe back pain, compared with those treated with alendronate [13, 24]. Teriparatide was more effective than other drugs in reducing back pain and improving the quality of life of postmenopausal osteoporotic women with VCFs [25]. The mechanism of back pain reduction likely includes a reduction in both severity and number of new VCFs [26] and improvement in bone microarchitecture and quality [13]. The VAS and JOA low back pain scores were significantly better after 6 months of treatment. After 6 months, the VAS continued to decrease, and the JOA score continued to increase; the difference between group A and group B was statistically significant at 12 and 18 months of treatment (p < 0.001).
Some biomechanical test data and clinical studies have suggested patients who undergo vertebroplasty or kyphoplasty had a greater risk of new VCFs compared with patients with prior VCFs who did not undergo either procedure [4]. Biomechanical test data demonstrated that fractured vertebrae treated with bone cement are stiffer than untreated vertebrae, and thus could transfer a greater load to adjacent vertebral levels [27, 28]. An increased fracture rate of the adjacent vertebrae has been observed after vertebroplasty [8]. Specifically, following vertebroplasty, patients are at increased risk of new-onset adjacent-level fractures and, when these fractures occur, they occur much sooner than non-adjacent-level fractures [6, 8].
Antiresorptive agents (alendronate, risedronate, raloxifene, and calcitonin) are widely used to treat osteoporosis. In a randomized trial of daily therapy with raloxifene for 24 months, the mean difference in the change in BMD between the women receiving 60 mg of raloxifene per day and those receiving a placebo was 2.4% ± 0.4% for the lumbar spine, 2.4% ± 0.4% for the total hip, and 2.0% ± 0.4% for the total body [29]. Treatment with 10 mg of alendronate daily for 10 years produced mean increases in BMD of 13.7% at the lumbar spine [30]. A randomized double-blind trial comparing the effect of teriparatide to that of alendronate sodium on BMD, non-vertebral fracture incidence, and bone turnover in 146 postmenopausal women with osteoporosis revealed that at 3 months, teriparatide was significantly more effective at increasing lumbar spine BMD than alendronate (p < 0.001). Lumbar spine BMD increased by 12.2% in the teriparatide group and 5.6% in the alendronate group after a mean treatment period of 14 months [31]. In our study, the percentage increase in lumbar spine BMD was 21.7% after 18 months of teriparatide treatment and 6.87% after 18 months of treatment with antiresorptive agents. Thus, the teriparatide-mediated BMD increase was much greater than that of antiresorptive therapy.
Currently, the extent to which the anti-fracture efficacy of antiresorptive drugs is related to changes in BMD is under debate. Wasnich and Miller have provided a model that predicted that treatments increasing spine BMD by 8% would reduce the risk of VCFs by 54% [32]. Data from clinical trials showed that raloxifene and alendronate reduced the risk of vertebral fracture by 40% to 50% after 3 years of treatment [9, 10]. Most new VCFs occurred within 3 months of PVP [6–8]. Although antiresorptive agents increased BMD and improved the bone quality of the lumbar spine, they were slow acting and did not rapidly increase BMD and guard against the development of new-onset VCFs after PVP.
Investigators have suggested that the gain in BMD with alendronate and other antiresorptive agents may be achieved by a remodeling of spaces, that is, reducing bone turnover without a true stimulation of bone formation [33]. Teriparatide (rDNA origin) injection (recombinant human parathyroid hormone, PTH [1–34]) directly stimulates bone formation via stimulating bone remodeling, increases BMD, and restores bone architecture and integrity. In contrast, bisphosphonates reduce bone resorption and increase BMD [31, 34]. Studies have shown that teriparatide induces large increases in biochemical markers of bone formation after 1 month of therapy, followed by a delayed increase in bone resorption markers [35]. These data show that teriparatide treatment for postmenopausal women with osteoporosis significantly increased cancellous bone volume and connectivity, improved trabecular morphology with a shift toward a more plate-like structure, and increased cortical bone thickness. These changes in cancellous and cortical bone morphology should improve biomechanical competence and are consistent with the substantially reduced incidences of vertebral and non-vertebral fractures during administration of teriparatide [36].
Two-dimensional histomorphometric and three-dimensional micro-computed tomography (CT) parameters were measured along with lumbar spine BMD at baseline and 12 or 18 months after teriparatide treatment. Since increases in BMD are correlated with improvements in trabecular microarchitecture in the iliac crests of patients taking teriparatide treatment, improvements in trabecular bone microarchitecture could be one of the mechanisms explaining how BMD increases improve bone strength during teriparatide treatment [37]. Teriparatide treatment results in an increase in BMD and is a potentially useful therapy for osteoporosis in men and for glucocorticoid-induced osteoporosis [38]. The subcutaneous daily dose of teriparatide (20 μg) decreased the occurrence of new VCFs in white women (70 years of age) by 65%, in a large randomized, double-blind, placebo-controlled trial. Moderate-to-severe fractures and multiple vertebral fractures were reduced by 90% and 77%, respectively. These results indicate that the clinical effects of teriparatide were consistent in both older and younger women. Age does not affect the safety and efficacy of teriparatide in postmenopausal women with osteoporosis [39]. In our study, teriparatide-mediated fracture risk reduction was 78.57%. Patients treated with teriparatide had a significantly lower risk of new-onset VCFs (OR = 0.21; 95% CI, 0.02–2.1).
In order to evaluate therapeutic effect, serial measurements of BMD are necessary. There is no absolutely reliable skeletal site or region of interest for monitoring these changes. The International Society for Clinical Densitometry recommends the lumbar spine as the most preferred bone site for monitoring serial changes in BMD [40, 41]. Even though one patient in group A and three patients in group B had only one usable vertebral body from L1 to L4 for the DEXA examination, we still preferred to use the lumbar spine for BMD monitoring of treatment.
Furthermore, the beneficial effects of teriparatide on vertebral fracture prevention and BMD persisted after treatment cessation. Teriparatide had a sustained effect in reducing the risk of non-vertebral fragility fractures for 18–30 months after discontinuation of treatment [42, 43].
As teriparatide is expensive, its use at the moment should be limited to patients with more severe forms of osteoporosis, usually with the presence or history of one or more fractures, because those patients are at high risk for subsequent fractures. We used teriparatide to treat new-onset adjacent VCFs after vertebroplasty and had good therapeutic pain relief and fracture prevention. Teriparatide is generally well tolerated, and treatment compliance rates are favorable. However, current limitations on the length of treatment and the high acquisition cost mean that teriparatide is best reserved for the treatment of patients with osteoporosis at high risk of fracture, or for patients with osteoporosis that have unsatisfactory responses to or intolerance of other osteoporosis therapies [38].
The limitations of the present study include the patient selection criteria. Some conditions, including degenerative lumbar spine disorder, long-term systemic disease, and previous leg fracture could affect the outcome of VCF treatment. Some patients in Taiwan seek out herbal medicines or folk remedies for back pain or other diseases, and some of these folk prescriptions include steroid, which can impact the therapeutic effect. Sometimes, patients suffering from a second VCF will seek out treatment in other hospitals. Many patients in our two groups had also undergone PVP in other hospitals and visited our hospital for medical treatment when new-onset VCFs occurred. Precise data on previous treatment for osteoporosis and medical history were difficult to obtain. The cost of teriparatide is high, and it is offered by Taiwan's Bureau of National Health Insurance for 18 months to severe osteoporostic patients. Only a few patients continued with self-paid treatment after the 18 months. Therefore, the treatment period was limited. Since the administration routes for teriparatide (subcutaneous injection) and antiresorptive agents (oral intake) are different, it is difficult to perform a double-blind study. An evaluation of pain relief should consider the dosage of analgesics, but complicated pharmacodynamics, side effects, and drug compliance with different analgesics may flaw the evaluation. The result of such studies would help patients choose the procedure that is optimal for them in terms of pain relief, fracture prevention, treatment safety and cost.
Conclusion
Fracture prevention and pain relief are the primary treatment goals for patients with osteoporotic VCFs. Although PVP can provide immediate pain relief, the procedure accelerates the failure rate in the adjacent vertebral body. Antiresorptive agents do not significantly and rapidly increase BMD and reduce the risk for VCFs. Most post-vertebroplasty new-onset adjacent VCFs occur within 2–3 months, and antiresorptive agents do not protect against their development. In our study, teriparatide-mediated BMD was significantly increased by 21.7% after 18 months of treatment, and fracture risk reduction was 78.57%. Teriparatide therapy significantly increased JOA and decreased VAS scores. The therapeutic effect of teriparatide is better than that of vertebroplasty combined with an antiresorptive treatment and is a potentially useful therapy for new-onset adjacent compression fractures after vertebroplasty.
References
Hall SE, Criddle RA, Comito TL, Prince RL (1999) A case-control study of quality of life and functional impairment in women with long-standing vertebral osteoporotic fracture. Osteoporos Int 9:508–515
Sambrook P, Cooper C (2006) Osteoporosis. Lancet 367:2010–2018
Ploeg WT, Veldhuizen AG, The B, Sietsma MS (2006) Percutaneous vertebroplasty as a treatment for osteoporotic vertebral compression fractures: a systematic review. Eur Spine J 15:1749–1758
Mudano AS, Bian J, Cope JU, Curtis JR, Gross TP, Allison JJ, Kim Y, Briggs D, Melton ME, Xi J, Saag KG (2009) Vertebroplasty and kyphoplasty are associated with an increased risk of secondary vertebral compression fractures: a population-based cohort study. Osteoporos Int 20:819–826
Boger A, Heini P, Windolf M, Schneider E (2007) Adjacent vertebral failure after vertebroplasty: a biomechanical study of low-modulus PMMA cement. Eur Spine J 16:2118–2125
Trout AT, Kallmes DF, Kaufmann TJ (2006) New fractures after vertebroplasty: adjacent fractures occur significantly sooner. Ajnr 27:217–223
Voormolen MH, Lohle PN, Juttmann JR, van der Graaf Y, Fransen H, Lampmann LE (2006) The risk of new osteoporotic vertebral compression fractures in the year after percutaneous vertebroplasty. J Vasc Interv Radiol 17:71–76
Tseng YY, Yang TC, Tu PH, Lo YL, Yang ST (2009) Repeated and multiple new vertebral compression fractures after percutaneous transpedicular vertebroplasty. Spine 34:1917–1922
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348:1535–1541
Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, Gluer CC, Krueger K, Cohen FJ, Eckert S, Ensrud KE, Avioli LV, Lips P, Cummings SR (1999) Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Jama 282:637–645
Kaufman JM, Orwoll E, Goemaere S, San Martin J, Hossain A, Dalsky GP, Lindsay R, Mitlak BH (2005) Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int 16:510–516
Marcus R, Wang O, Satterwhite J, Mitlak B (2003) The skeletal response to teriparatide is largely independent of age, initial bone mineral density, and prevalent vertebral fractures in postmenopausal women with osteoporosis. J Bone Miner Res 18:18–23
Arlot M, Meunier PJ, Boivin G, Haddock L, Tamayo J, Correa-Rotter R, Jasqui S, Donley DW, Dalsky GP, Martin JS, Eriksen EF (2005) Differential effects of teriparatide and alendronate on bone remodeling in postmenopausal women assessed by histomorphometric parameters. J Bone Miner Res 20:1244–1253
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148
Scott PJ, Huskisson EC (1977) Measurement of functional capacity with visual analogue scales. Rheumatol Rehabil 16:257–259
Fukui M, Chiba K, Kawakami M, Kikuchi S, Konno S, Miyamoto M, Seichi A, Shimamura T, Shirado O, Taguchi T, Takahashi K, Takeshita K, Tani T, Toyama Y, Yonenobu K, Wada E, Tanaka T, Hirota Y (2007) Japanese Orthopaedic Association Back Pain Evaluation Questionnaire. Part 2. Verification of its reliability: The Subcommittee on Low Back Pain and Cervical Myelopathy Evaluation of the Clinical Outcome Committee of the Japanese Orthopaedic Association. J Orthop Sci 12:526–532
Majumdar SR, Kim N, Colman I, Chahal AM, Raymond G, Jen H, Siminoski KG, Hanley DA, Rowe BH (2005) Incidental vertebral fractures discovered with chest radiography in the emergency department: prevalence, recognition, and osteoporosis management in a cohort of elderly patients. Arch Intern Med 165:905–909
Buchbinder R, Osborne RH, Ebeling PR, Wark JD, Mitchell P, Wriedt C, Graves S, Staples MP, Murphy B (2009) A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. The New Engl J Med 361:557–568
Buchbinder R, Osborne RH, Kallmes D (2009) Vertebroplasty appears no better than placebo for painful osteoporotic spinal fractures, and has potential to cause harm. The Med J Australia 191:476–477
Kallmes DF, Comstock BA, Heagerty PJ, Turner JA, Wilson DJ, Diamond TH, Edwards R, Gray LA, Stout L, Owen S, Hollingworth W, Ghdoke B, Annesley-Williams DJ, Ralston SH, Jarvik JG (2009) A randomized trial of vertebroplasty for osteoporotic spinal fractures. The New Engl J Med 361:569–579
Lin CC, Shen WC, Lo YC, Liu YJ, Yu TC, Chen IH, Chung HW (2010) Recurrent pain after percutaneous vertebroplasty. Ajr 194:1323–1329
Nevitt MC, Chen P, Kiel DP, Reginster JY, Dore RK, Zanchetta JR, Glass EV, Krege JH (2006) Reduction in the risk of developing back pain persists at least 30 months after discontinuation of teriparatide treatment: a meta-analysis. Osteoporos Int 17:1630–1637
Nevitt MC, Chen P, Dore RK, Reginster JY, Kiel DP, Zanchetta JR, Glass EV, Krege JH (2006) Reduced risk of back pain following teriparatide treatment: a meta-analysis. Osteoporos Int 17:273–280
McClung MR, San Martin J, Miller PD, Civitelli R, Bandeira F, Omizo M, Donley DW, Dalsky GP, Eriksen EF (2005) Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med 165:1762–1768
Ulivieri FM (2007) Back pain treatment in post-menopausal osteoporosis with vertebral fractures. Aging Clin Exp Res 19:21–23
Genant HK, Halse J, Briney WG, Xie L, Glass EV, Krege JH (2005) The effects of teriparatide on the incidence of back pain in postmenopausal women with osteoporosis. Curr Med Res Opin 21:1027–1034
Polikeit A, Nolte LP, Ferguson SJ (2003) The effect of cement augmentation on the load transfer in an osteoporotic functional spinal unit: finite-element analysis. Spine 28:991–996
Nouda S, Tomita S, Kin A, Kawahara K, Kinoshita M (2009) Adjacent vertebral body fracture following vertebroplasty with polymethylmethacrylate or calcium phosphate cement: biomechanical evaluation of the cadaveric spine. Spine 34:2613–2618
Delmas PD, Bjarnason NH, Mitlak BH, Ravoux AC, Shah AS, Huster WJ, Draper M, Christiansen C (1997) Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. The New Engl J Med 337:1641–1647
Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J, Santora AC, Liberman UA (2004) Ten years' experience with alendronate for osteoporosis in postmenopausal women. The New Engl J Med 350:1189–1199
Body JJ, Gaich GA, Scheele WH, Kulkarni PM, Miller PD, Peretz A, Dore RK, Correa-Rotter R, Papaioannou A, Cumming DC, Hodsman AB (2002) A randomized double-blind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1–34)] with alendronate in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 87:4528–4535
Wasnich RD, Miller PD (2000) Antifracture efficacy of antiresorptive agents are related to changes in bone density. J Clin Endocrinol Metab 85:231–236
Hernandez CJ, Beaupre GS, Marcus R, Carter DR (2001) A theoretical analysis of the contributions of remodeling space, mineralization, and bone balance to changes in bone mineral density during alendronate treatment. Bone 29:511–516
Chen P, Miller PD, Delmas PD, Misurski DA, Krege JH (2006) Change in lumbar spine BMD and vertebral fracture risk reduction in teriparatide-treated postmenopausal women with osteoporosis. J Bone Miner Res 21:1785–1790
Glover SJ, Eastell R, McCloskey EV, Rogers A, Garnero P, Lowery J, Belleli R, Wright TM, John MR (2009) Rapid and robust response of biochemical markers of bone formation to teriparatide therapy. Bone 45:1053–1058
Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF (2003) Recombinant human parathyroid hormone (1–34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res 18:1932–1941
Chen P, Miller PD, Recker R, Resch H, Rana A, Pavo I, Sipos AA (2007) Increases in BMD correlate with improvements in bone microarchitecture with teriparatide treatment in postmenopausal women with osteoporosis. J Bone Miner Res 22:1173–1180
Blick SK, Dhillon S, Keam SJ (2008) Teriparatide: a review of its use in osteoporosis. Drugs 68:2709–2737
Boonen S, Marin F, Mellstrom D, Xie L, Desaiah D, Krege JH, Rosen CJ (2006) Safety and efficacy of teriparatide in elderly women with established osteoporosis: bone anabolic therapy from a geriatric perspective. J Am Geriatr Soc 54:782–789
Abrahamsen B, Hansen TB, Jensen LB, Hermann AP, Eiken P (1997) Site of osteodensitometry in perimenopausal women: correlation and limits of agreement between anatomic regions. J Bone Miner Res 12:1471–1479
Miller PD, Zapalowski C, Kulak CA, Bilezikian JP (1999) Bone densitometry: the best way to detect osteoporosis and to monitor therapy. J Clin Endocrinol Metab 84:1867–1871
Prince R, Sipos A, Hossain A, Syversen U, Ish-Shalom S, Marcinowska E, Halse J, Lindsay R, Dalsky GP, Mitlak BH (2005) Sustained nonvertebral fragility fracture risk reduction after discontinuation of teriparatide treatment. J Bone Miner Res 20:1507–1513
Lindsay R, Scheele WH, Neer R, Pohl G, Adami S, Mautalen C, Reginster JY, Stepan JJ, Myers SL, Mitlak BH (2004) Sustained vertebral fracture risk reduction after withdrawal of teriparatide in postmenopausal women with osteoporosis. Arch Intern Med 164:2024–2030
Conflicts of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
The drugs are FDA-approved for this indication, and no medical device was used in the research for this manuscript. No funds were received in support of this work. No benefit in any form has been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Tseng, YY., Su, CH., Lui, TN. et al. Prospective comparison of the therapeutic effect of teriparatide with that of combined vertebroplasty with antiresorptive agents for the treatment of new-onset adjacent vertebral compression fracture after percutaneous vertebroplasty. Osteoporos Int 23, 1613–1622 (2012). https://doi.org/10.1007/s00198-011-1730-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-011-1730-y