Abstract
Summary
Genetic hemochromatosis is a cause of osteoporosis; mechanisms leading to iron-related bone loss are not fully characterized. We assessed the bone phenotype of HFE −/− male mice, a mouse model of hemochromatosis. They had a phenotype of osteoporosis with low bone mass and alteration of the bone microarchitecture.
Introduction
Genetic hemochromatosis is a cause of osteoporosis. However, the mechanisms leading to iron-related bone loss are not fully characterized. Recent human data have not supported the hypothesis of hypogonadism involvement. The direct role of iron on bone metabolism has been suggested.
Methods
Our aim was to assess the bone phenotype of HFE −/− male mice, a mouse model of human hemochromatosis, by using microcomputed tomography and histomorphometry. HFE −/− animals were sacrificed at 6 and 12 months and compared to controls.
Results
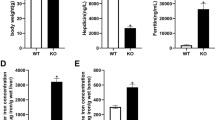
There was a significant increase in hepatic iron concentration and bone iron content in HFE −/− mice. No detectable Perls’ staining was found in the controls’ trabeculae. Trabecular bone volume (BV/TV) was significantly lower in HFE −/− mice at 6 and 12 months compared to the corresponding wild-type mice: 9.88 ± 0.82% vs 12.82 ± 0.61% (p = 0.009) and 7.18 ± 0.68% vs 10.4 ± 0.86% (p = 0.015), respectively. In addition, there was an impairment of the bone microarchitecture in HFE −/− mice. Finally, we found a significant increase in the osteoclast number in HFE −/− mice: 382.5 ± 36.75 vs 273.4 ± 20.95 ¢/mm2 (p = 0.004) at 6 months and 363.6 ± 22.35 vs 230.8 ± 18.7 ¢/mm2 (p = 0.001) at 12 months in HFE −/− mice vs controls.
Conclusion
Our data show that HFE −/− male mice develop a phenotype of osteoporosis with low bone mass and alteration of the microarchitecture. They suggest that there is a relationship between bone iron overload and the increase of the osteoclast number in these mice. These findings are in accordance with clinical observations in humans exhibiting genetic hemochromatosis and support a role of excess iron in relation to genetic hemochromatosis in the development of osteoporosis in humans.



Similar content being viewed by others
References
Delbarre F (1960) Osteoporosis in hemochromatosis. Sem Hôp 36:3279–3294
Niederau C, Fischer R, Purschel A, Stremmel W, Haussinger D, Strohmeyer G (1996) Long-term survival in patients with hereditary hemochromatosis. Gastroenterology 110:1107–1119
Brissot P, Troadec MB, Bardou-Jacquet E, Le Lan C, Jouanolle AM, Deugnier Y, Loreal O (2008) Current approach to hemochromatosis. Blood Rev 22:195–210
Pawlotsky Y, Le Dantec P, Moirand R, Guggenbuhl P, Jouanolle AM, Catheline M, Meadeb J, Brissot P, Deugnier Y, Chales G (1999) Elevated parathyroid hormone 44–68 and osteoarticular changes in patients with genetic hemochromatosis. Arthritis Rheum 42:799–806
Guggenbuhl P, Albert JD, Chales G (2007) Rheumatic manifestations of genetic hemochromatosis. Presse Méd 36:1313–1318
Sinigaglia L, Fargion S, Fracanzani AL, Binelli L, Battafarano N, Varenna M, Piperno A, Fiorelli G (1997) Bone and joint involvement in genetic hemochromatosis: role of cirrhosis and iron overload. J Rheumatol 24:1809–1813
Guggenbuhl P, Deugnier Y, Boisdet JF, Rolland Y, Perdriger A, Pawlotsky Y, Chales G (2005) Bone mineral density in men with genetic hemochromatosis and HFE gene mutation. Osteoporos Int 16:1809–1814
Pawlotsky Y, Lancien Y, Roudier G, Hany Y, Louboutin JY, Ferrand B, Bourel M (1979) Bone histomorphometry and osteo-articular manifestations of idiopathic hemochromatosis. Rev Rhum Mal Osteo-artic 46:91–99
Bisbocci D, Marinone C, Ballanti P, Villata E, Suriano A, Vallauri P, Chiandussi L (1991) Bone involvement in primary hemochromatosis: osteoporosis and hormonal bone pattern in hemochromatosis. In: Gentilini P, Dianzani MU (eds) Experimental and clinical hepatology. Elsevier Science, London, pp 163–169
de Vernejoul MC, Pointillart A, Golenzer CC, Morieux C, Bielakoff J, Modrowski D, Miravet L (1984) Effects of iron overload on bone remodeling in pigs. Am J Pathol 116:377–384
Conte D, Caraceni MP, Duriez J, Mandelli C, Corghi E, Cesana M, Ortolani S, Bianchi PA (1989) Bone involvement in primary hemochromatosis and alcoholic cirrhosis. Am J Gastroenterol 84:1231–1234
Diamond TH, Stiel D, Lunzer M, McDowall D, Eckstein RP, Posen S (1989) Hepatic osteodystrophy. Static and dynamic bone histomorphometry and serum bone Gla-protein in 80 patients with chronic liver disease. Gastroenterology 96:213–221
Angelopoulos NG, Goula AK, Papanikolaou G, Tolis G (2006) Osteoporosis in HFE2 juvenile hemochromatosis. A case report and review of the literature. Osteoporos Int 17:150–155
Jensen CE, Tuck SM, Agnew JE, Koneru S, Morris RW, Yardumian A, Prescott E, Hoffbrand AV, Wonke B (1998) High prevalence of low bone mass in thalassaemia major. Br J Haematol 103:911–915
Weinberg ED (2006) Iron loading: a risk factor for osteoporosis. Biometals 19:633–635
Dupic F, Fruchon S, Bensaid M, Borot N, Radosavljevic M, Loreal O, Brissot P, Gilfillan S, Bahram S, Coppin H, Roth MP (2002) Inactivation of the hemochromatosis gene differentially regulates duodenal expression of iron-related mRNAs between mouse strains. Gastroenterology 122:745–751
Klinck J, Boyd SK (2008) The magnitude and rate of bone loss in ovariectomized mice differs among inbred strains as determined by longitudinal in vivo micro-computed tomography. Calcif Tissue Int 83:70–79
Chappard D, Basle MF, Legrand E, Audran M (2008) Trabecular bone microarchitecture: a review. Morphologie 92:162–170
Chappard D (2009) Technical aspects: how do we best prepare bone samples for proper histological analysis? In D H (ed) Bone cancer: progression and therapeutic approaches. Academic Press; Elsevier, London, pp 203–210
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610
Chappard D (1990) Osteoclast count on human bone biopsies: Why and How? In: Takahashi FE (ed) Bone morphometry. Nishimura-Smith-Gordon, Niigata, London, pp 248–255
Barry M, Sherlock S (1971) Measurement of liver-iron concentration in needle-biopsy specimens. Lancet 1:100–103
Matsushima S, Hoshimoto M, Torii M, Ozaki K, Narama I (2001) Iron lactate-induced osteopenia in male Sprague-Dawley rats. Toxicol Pathol 29:623–629
Yamasaki K, Hagiwara H (2009) Excess iron inhibits osteoblast metabolism. Toxicol Lett 191:211–215
Messer JG, Kilbarger AK, Erikson KM, Kipp DE (2009) Iron overload alters iron-regulatory genes and proteins, down-regulates osteoblastic phenotype, and is associated with apoptosis in fetal rat calvaria cultures. Bone 45:972–979
Zarjou A, Jeney V, Arosio P, Poli M, Zavaczki E, Balla G, Balla J (2010) Ferritin-ferroxidase activity: a potent inhibitor of osteogenesis. J Bone Miner Res 25:164–172
Guggenbuhl P, Filmon R, Mabilleau G, Basle MF, Chappard D (2008) Iron inhibits hydroxyapatite crystal growth in vitro. Metabolism 57:903–910
Nakagawa H, Wachi M, Woo JT, Kato M, Kasai S, Takahashi F, Lee IS, Nagai K (2002) Fenton reaction is primarily involved in a mechanism of (-)-epigallocatechin-3-gallate to induce osteoclastic cell death. Biochem Biophys Res Commun 292:94–101
Tsay J, Yang Z, Ross FP, Cunningham-Rundles S, Lin H, Coleman R, Mayer-Kuckuk P, Doty SB, Grady RW, Giardina PJ, Boskey AL, Vogiatzi MG (2010) Bone loss due to iron overload in a murine model: importance of oxidative stress. Blood 116:2582–2589
Zaidi M (2007) Skeletal remodeling in health and disease. Nat Med 13:791–801
Edwards CM, Mundy GR (2008) Eph receptors and ephrin signaling pathways: a role in bone homeostasis. Int J Med Sci 5:263–272
Isomura H, Fujie K, Shibata K, Inoue N, Iizuka T, Takebe G, Takahashi K, Nishihira J, Izumi H, Sakamoto W (2004) Bone metabolism and oxidative stress in postmenopausal rats with iron overload. Toxicology 197:93–100
Morabito N, Russo GT, Gaudio A, Lasco A, Catalano A, Morini E, Franchina F, Maisano D, La Rosa M, Plota M, Crifo A, Meo A, Frisina N (2007) The “lively” cytokines network in beta-Thalassemia Major-related osteoporosis. Bone 40:1588–1594
Morabito N, Gaudio A, Lasco A, Atteritano M, Pizzoleo MA, Cincotta M, La Rosa M, Guarino R, Meo A, Frisina N (2004) Osteoprotegerin and RANKL in the pathogenesis of thalassemia-induced osteoporosis: new pieces of the puzzle. J Bone Miner Res 19:722–727
Mahachoklertwattana P, Sirikulchayanonta V, Chuansumrit A, Karnsombat P, Choubtum L, Sriphrapradang A, Domrongkitchaiporn S, Sirisriro R, Rajatanavin R (2003) Bone histomorphometry in children and adolescents with beta-thalassemia disease: iron-associated focal osteomalacia. J Clin Endocrinol Metab 88:3966–3972
Acknowledgments
This study was funded by grants from the Société Française de Rhumatologie (SFR) and the Région Bretagne (MD).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guggenbuhl, P., Fergelot, P., Doyard, M. et al. Bone status in a mouse model of genetic hemochromatosis. Osteoporos Int 22, 2313–2319 (2011). https://doi.org/10.1007/s00198-010-1456-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-010-1456-2