Abstract
Summary
Findings from this 5-year phase 3 study of postmenopausal women with osteoporosis showed that bazedoxifene was associated with an overall favorable safety and tolerability profile, with no evidence of endometrial or breast stimulation. Overall, the results at 5 years were consistent with those seen at 3 years.
Introduction
We report safety and tolerability findings from a 5-year randomized, double-blind, phase 3 study of bazedoxifene in postmenopausal women with osteoporosis.
Methods
In the core study, healthy postmenopausal women with osteoporosis (N = 7,492; mean age, 66.4 years) were randomized to daily doses of bazedoxifene 20 or 40 mg, raloxifene 60 mg, or placebo for 3 years. During the 2-year study extension, the raloxifene 60-mg treatment arm was discontinued after the 3-year database was finalized, and subjects receiving bazedoxifene 40 mg were transitioned in a blinded manner to bazedoxifene 20 mg (bazedoxifene 40-/20-mg group) after 4 years. Safety and tolerability data are reported for subjects in the bazedoxifene 20- and 40-/20-mg and placebo groups; efficacy findings are reported elsewhere.
Results
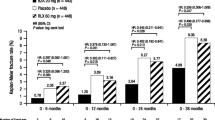
A total of 3,146 subjects in the bazedoxifene 20- and 40-mg and placebo groups were enrolled in the extension study (years 4 and 5). Overall, the 5-year incidence of adverse events (AEs), serious AEs, and discontinuations due to AEs were similar among groups. The incidence of hot flushes and leg cramps was higher with bazedoxifene compared with placebo. Venous thromboembolic events, primarily deep vein thrombosis, were more frequently reported in the bazedoxifene groups compared with the placebo group. Reports of cardiac disorders and cerebrovascular events were few and evenly distributed among groups. Bazedoxifene showed a neutral effect on the breast and endometrium.
Conclusion
Bazedoxifene was associated with an overall favorable safety and tolerability profile in postmenopausal women with osteoporosis over 5 years of therapy, consistent with findings at 3 years.

Similar content being viewed by others
References
North American Menopause Society (2006) Management of osteoporosis in postmenopausal women: 2006 position statement of The North American Menopause Society. Menopause 13:340–367
Fontana A, Delmas PD (2003) Selective estrogen receptors modulators in the prevention and treatment of postmenopausal osteoporosis. Endocrinol Metab Clin N Am 32:219–232
Dennison E, Cooper C (2000) Epidemiology of osteoporotic fractures. Horm Res 54:58–63
Atik OS, Gunal I, Korkusuz F (2006) Burden of osteoporosis. Clin Orthop Relat Res 443:19–24
Caliri A, De Filippis L, Bagnato GL, Bagnato GF (2007) Osteoporotic fractures: mortality and quality of life. Panminerva Med 49:21–27
MacLean C, Newberry S, Maglione M, McMahon M, Ranganath V, Suttorp M, Mojica W, Timmer M, Alexander A, McNamara M, Desai SB, Zhou A, Chen S, Carter J, Tringale C, Valentine D, Johnsen B, Grossman J (2008) Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med 148:197–213
Lewiecki EM (2009) Emerging drugs for postmenopausal osteoporosis. Expert Opin Emerg Drugs 14:129–144
Arun B, Anthony M, Dunn B (2002) The search for the ideal SERM. Expert Opin Pharmacother 3:681–691
Taylor HS (2009) Designing the ideal selective estrogen receptor modulator—an achievable goal? Menopause 16:609–615
Cranney A, Adachi JD (2005) Benefit-risk assessment of raloxifene in postmenopausal osteoporosis. Drug Saf 28:721–730
Goldstein SR, Nanavati N (2002) Adverse events that are associated with the selective estrogen receptor modulator levormeloxifene in an aborted phase III osteoporosis treatment study. Am J Obstet Gynecol 187:521–527
Shelly W, Draper MW, Krishnan V, Wong M, Jaffe RB (2008) Selective estrogen receptor modulators: an update on recent clinical findings. Obstet Gynecol Surv 63:163–181
Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, McNabb MA, Wenger NK (2006) Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 355:125–137
Grady D, Ettinger B, Moscarelli E, Plouffe L Jr, Sarkar S, Ciaccia A, Cummings S (2004) Safety and adverse effects associated with raloxifene: multiple outcomes of raloxifene evaluation. Obstet Gynecol 104:837–844
Martino S, Cauley JA, Barrett-Connor E, Powles TJ, Mershon J, Disch D, Secrest RJ, Cummings SR (2004) Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst 96:1751–1761
Miller PD, Chines AA, Christiansen C, Hoeck HC, Kendler DL, Lewiecki EM, Woodson G, Levine AB, Constantine G, Delmas PD (2008) Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-yr results of a randomized, double-blind, placebo-, and active-controlled study. J Bone Miner Res 23:525–535
Pinkerton JV, Archer DF, Utian WH, Menegoci JC, Levine AB, Chines AA, Constantine GD (2009) Bazedoxifene effects on the reproductive tract in postmenopausal women at risk for osteoporosis. Menopause 16:1102–1108
Silverman SL, Christiansen C, Genant HK, Vukicevic S, Zanchetta JR, de Villiers TJ, Constantine GD, Chines AA (2008) Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res 23:1923–1934
Archer DF, Pinkerton JV, Utian WH, Menegoci JC, de Villiers TJ, Yuen CK, Levine AB, Chines AA, Constantine GD (2009) Bazedoxifene, a selective estrogen receptor modulator: effects on the endometrium, ovaries, and breast from a randomized controlled trial in osteoporotic postmenopausal women. Menopause 16:1109–1115
Silverman S, Chines A, Zanchetta JR, Genant H, Kendler D, Rio de la Loza F, Kung A, Constantine GD, Adachi J (2009) Sustained efficacy of bazedoxifene in preventing fractures in postmenopausal women with osteoporosis: results of a 5-year, randomized, placebo-controlled study. J Bone Miner Res 24 (Suppl 1). Available at: http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=2643ea2f-c4ab-4eb6-9798-09050bb21b6b. Accessed 10 Dec 2009
Davies GC, Huster WJ, Lu Y, Plouffe L Jr, Lakshmanan M (1999) Adverse events reported by postmenopausal women in controlled trials with raloxifene. Obstet Gynecol 93:558–565
Pfizer Inc (2008) FABLYN® (lasofoxifene tartrate) 0.5 mg tablets. NDA 22-242. Reproductive Health Drugs Advisory Committee Briefing Document, 08 September 2008. http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4381b1-02-Pfizer.pdf. Accessed 10 Dec 2009
Fleischer AC, Wheeler JE, Yeh IT, Kravitz B, Jensen C, MacDonald B (1999) Sonographic assessment of the endometrium in osteopenic postmenopausal women treated with idoxifene. J Ultrasound Med 18:503–512
Alexandersen P, Riis BJ, Stakkestad JA, Delmas PD, Christiansen C (2001) Efficacy of levormeloxifene in the prevention of postmenopausal bone loss and on the lipid profile compared to low dose hormone replacement therapy. J Clin Endocrinol Metab 86:755–760
SmithKline Beecham (1999) SmithKline Beecham drops idoxifene for osteoporosis. SCRIP 2431:21
Novo Nordisk (1998) Novo Nordisk drops levormeloxifene. SCRIP 2374:18
Ronkin S, Northington R, Baracat E, Nunes MG, Archer DF, Constantine G, Pickar JH (2005) Endometrial effects of bazedoxifene acetate, a novel selective estrogen receptor modulator, in postmenopausal women. Obstet Gynecol 105:1397–1404
Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N (1998) Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 90:1371–1388
Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, Gluer CC, Krueger K, Cohen FJ, Eckert S, Ensrud KE, Avioli LV, Lips P, Cummings SR, for the Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators (1999) Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA 282:637–645
Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, Norton L, Nickelsen T, Bjarnason NH, Morrow M, Lippman ME, Black D, Glusman JE, Costa A, Jordan VC (1999) The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA 281:2189–2197
Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, Bevers TB, Fehrenbacher L, Pajon ER Jr, Wade JL III, Robidoux A, Margolese RG, James J, Lippman SM, Runowicz CD, Ganz PA, Reis SE, Caskill-Stevens W, Ford LG, Jordan VC, Wolmark N (2006) Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 295:2727–2741
Acknowledgments
This study was sponsored by Wyeth, which was acquired by Pfizer Inc in October 2009. Medical writing support for this manuscript was provided by Bo Choi, Ph.D., of MedErgy and was funded by Wyeth. Members of the independent adjudication boards included Geno Merli, MD, of Thomas Jefferson Hospital; Michael Ezekowitz, MD, Ph.D., of Lankenau Hospital; George Tzanis, MD, of Thomas Jefferson Hospital; Linda Bagley, MD, of Penn Radiology at Pennsylvania Hospital; Rodney Bell, MD, of Thomas Jefferson Hospital; and Scott Kasner, MD, of the University of Pennsylvania Hospital.
Conflicts of interest
Dr. de Villiers has served as an advisory board member and/or consultant for MSD, Novartis, Servier, and Wyeth. Dr. Palacios has been a symposium speaker or advisory board member for Bayer-Schering, Novo Nordisk, Servier, Lilly, Daiichi-Sankyo, Sanofi-Aventis, MSD, and Procter & Gamble. He has also received research grants and/or consulting fees from Wyeth, Servier, Lilly, Daiichi-Sankyo, Amgen, Arkochim, and Bayer-Schering. Dr. Lips has received research grants and/or honoraria from Eli Lilly, Merck, Procter & Gamble, Servier, and Wyeth. Dr. Brown is an investigator for Wyeth and has served as a consultant for and/or received honoraria or research funding from Abbott, Amgen, Arthrolab, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Merck Frosst, Novartis, Nycomed, Pfizer, Procter & Gamble, Roche, Sanofi-Aventis, Servier, and Wyeth. Drs. Chines, Levine, and Kelepouris were employees of Wyeth, which was acquired by Pfizer Inc in October 2009.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Villiers, T.J., Chines, A.A., Palacios, S. et al. Safety and tolerability of bazedoxifene in postmenopausal women with osteoporosis: results of a 5-year, randomized, placebo-controlled phase 3 trial. Osteoporos Int 22, 567–576 (2011). https://doi.org/10.1007/s00198-010-1302-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-010-1302-6