Abstract
Summary
This 6-month study examined the efficacy and safety of bazedoxifene 20 mg in postmenopausal Asian women. Bazedoxifene showed statistically significant improvements over placebo in bone mineral density at all skeletal sites evaluated. Bazedoxifene significantly reduced bone turnover and had favorable effects on lipid parameters. Bazedoxifene was safe and well tolerated.
Introduction
This 6-month, randomized, double-blind, placebo-controlled phase 3 study conducted in China, Korea, and Taiwan evaluated the efficacy and safety of bazedoxifene in postmenopausal Asian women.
Methods
Generally, healthy postmenopausal Asian women (N = 487; mean age, 57.2 years; mean lumbar spine bone mineral density [BMD], −1.1) were randomized to daily therapy with bazedoxifene 20 mg or placebo; all subjects received daily supplemental calcium carbonate 600 mg. The changes from baseline in BMD at the lumbar spine (primary end point) and at other skeletal sites, bone turnover markers, and lipid parameters were evaluated at 6 months. Safety assessments included adverse event (AE) reporting and physical/gynecologic examination.
Results
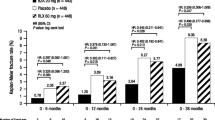
At 6 months, women who received bazedoxifene 20 mg had significantly greater BMD compared with those receiving placebo at the lumbar spine (0.41% vs −0.32%, P < 0.01), femoral neck (−0.08% vs −0.69%, P = 0.014), trochanter (0.50% vs −0.23%, P = 0.010), and total hip (−0.03% vs −0.77%, P < 0.001), respectively. Bazedoxifene 20 mg was also associated with significant differences from placebo in median percent reductions from baseline in serum C-telopeptide (−21.8%, P < 0.001) and osteocalcin (−12.9%, P < 0.001) levels and total (−5.0%, P < 0.001) and low-density lipoprotein cholesterol (−9.5%, P < 0.001) levels. The incidence of AEs was not different between subjects treated with bazedoxifene and those who received placebo.
Conclusion
Bazedoxifene was generally safe and effective in preventing bone loss in this short-term study of postmenopausal Asian women.


Similar content being viewed by others
References
Cole Z, Dennison E, Cooper C (2008) Update on the treatment of post-menopausal osteoporosis. Br Med Bull 86:129–143
Garnero P (2008) Biomarkers for osteoporosis management: utility in diagnosis, fracture risk prediction and therapy monitoring. Mol Diagn Ther 12:157–170
Dennison E, Cooper C (2000) Epidemiology of osteoporotic fractures. Horm Res 54:58–63
Finkelstein JS, Brockwell SE, Mehta V, Greendale GA, Sowers MR, Ettinger B, Lo JC, Johnston JM, Cauley JA, Danielson ME, Neer RM (2008) Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab 93:861–868
Nakamura T, Liu JL, Morii H, Huang QR, Zhu HM, Qu Y, Hamaya E, Thiebaud D (2006) Effect of raloxifene on clinical fractures in Asian women with postmenopausal osteoporosis. J Bone Miner Metab 24:414–418
Wu XP, Liao EY, Zhang H, Dai RC, Shan PF, Cao XZ, Liu SP, Jiang Y (2004) Determination of age-specific bone mineral density and comparison of diagnosis and prevalence of primary osteoporosis in Chinese women based on both Chinese and World Health Organization criteria. J Bone Miner Metab 22:382–391
North American Menopause Society (2006) Management of osteoporosis in postmenopausal women: 2006 position statement of The North American Menopause Society. Menopause 13:340–367
Grey A (2007) Emerging pharmacologic therapies for osteoporosis. Expert Opin Emerg Drugs 12:493–508
Tosteson AN, Melton LJ III, Dawson-Hughes B, Baim S, Favus MJ, Khosla S, Lindsay RL (2008) Cost-effective osteoporosis treatment thresholds: the United States perspective. Osteoporos Int 19:437–447
Koh LK (2002) An Asian perspective to the problem of osteoporosis. Ann Acad Med Singapore 31:26–29
Hosking D, Chilvers CE, Christiansen C, Ravn P, Wasnich R, Ross P, McClung M, Balske A, Thompson D, Daley M, Yates AJ, for the Early Postmenopausal Intervention Cohort Study Group (1998) Prevention of bone loss with alendronate in postmenopausal women under 60 years of age. N Engl J Med 338:485–492
Wasnich RD, Ross PD, Thompson DE, Cizza G, Yates AJ (1999) Skeletal benefits of two years of alendronate treatment are similar for early postmenopausal Asian and Caucasian women. Osteoporos Int 9:455–460
Kung A, Chao HT, Huang KE, Need AG, Taechakraichana N, Loh FH, Gonzaga F, Sriram U, Ismail NM, Farooqu A, Rachman IA, Crans GG, Wong M, Thiebaud D (2003) Efficacy and safety of raloxifene 60 milligrams/day in postmenopausal Asian women. J Clin Endocrinol Metab 88:3130–3136
Miller PD, Chines AA, Christiansen C, Hoeck HC, Kendler DL, Lewiecki EM, Woodson G, Levine AB, Constantine G, Delmas PD (2008) Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-yr results of a randomized, double-blind, placebo-, and active-controlled study. J Bone Miner Res 23:525–535
Silverman SL, Christiansen C, Genant HK, Vukicevic S, Zanchetta JR, de Villiers TJ, Constantine GD, Chines AA (2008) Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo- and active-controlled clinical trial. J Bone Miner Res 23:1923–1934
Delmas PD, Bjarnason NH, Mitlak BH, Ravoux AC, Shah AS, Huster WJ, Draper M, Christiansen C (1997) Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med 337:1641–1647
Jolly EE, Bjarnason NH, Neven P, Plouffe L Jr, Johnston CC Jr, Watts SD, Arnaud CD, Mason TM, Crans G, Akers R, Draper MW (2003) Prevention of osteoporosis and uterine effects in postmenopausal women taking raloxifene for 5 years. Menopause 10:337–344
Hooper MJ, Ebeling PR, Roberts AP, Graham JJ, Nicholson GC, D'Emden M, Ernst TF, Wenderoth D (2005) Risedronate prevents bone loss in early postmenopausal women: a prospective randomized, placebo-controlled trial. Climacteric 8:251–262
Mortensen L, Charles P, Bekker PJ, Digennaro J, Johnston CC Jr (1998) Risedronate increases bone mass in an early postmenopausal population: two years of treatment plus one year of follow-up. J Clin Endocrinol Metab 83:396–402
Ravn P, Weiss SR, Rodriguez-Portales JA, McClung MR, Wasnich RD, Gilchrist NL, Sambrook P, Fogelman I, Krupa D, Yates AJ, Daifotis A, Fuleihan GE (2000) Alendronate in early postmenopausal women: effects on bone mass during long-term treatment and after withdrawal. Alendronate Osteoporosis Prevention Study Group. J Clin Endocrinol Metab 85:1492–1497
Itabashi A, Ohta H, Ebede B, Constantine G, Chines AA (2008) A double-blind, placebo-controlled, dose-response study of bazedoxifene in Japanese postmenopausal women with osteoporosis. In: 2008 American Society for Bone and Mineral Research Abstract Book. Washington, DC: American Society for Bone and Mineral Research; 2008: 472. Abstract M391
Arun B, Anthony M, Dunn B (2002) The search for the ideal SERM. Expert Opin Pharmacother 3:681–691
Gold EB, Sternfeld B, Kelsey JL, Brown C, Mouton C, Reame N, Salamone L, Stellato R (2000) Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40–55 years of age. Am J Epidemiol 152:463–473
Chim H, Tan BH, Ang CC, Chew EM, Chong YS, Saw SM (2002) The prevalence of menopausal symptoms in a community in Singapore. Maturitas 41:275–282
Shea JL (2006) Chinese women's symptoms: relation to menopause, age and related attitudes. Climacteric 9:30–39
Melby MK, Lock M, Kaufert P (2005) Culture and symptom reporting at menopause. Hum Reprod Update 11:495–512
Rice VM (2005) Strategies and issues for managing menopause-related symptoms in diverse populations: ethnic and racial diversity. Am J Med 118 Suppl:142–147
Crandall CJ, Crawford SL, Gold EB (2006) Vasomotor symptom prevalence is associated with polymorphisms in sex steroid-metabolizing enzymes and receptors. Am J Med 119:S52–S60
Neff MJ (2004) NAMS releases position statement on the treatment of vasomotor symptoms associated with menopause. Am Fam Physician 70:393–394, 396, 399
Sugimoto T, Itabashi A, Ohta H, Ochi H, Chines AA, Constantine G (2008) The safety profile of bazedoxifene in Japanese postmenopausal women with osteoporosis. In: 2008 American Society for Bone and Mineral Research Abstract Book. Washington, DC: American Society for Bone and Mineral Research; 2008: 471. Abstract M389
Ravn P, Hosking D, Thompson D, Cizza G, Wasnich RD, McClung M, Yates AJ, Bjarnason NH, Christiansen C (1999) Monitoring of alendronate treatment and prediction of effect on bone mass by biochemical markers in the early postmenopausal intervention cohort study. J Clin Endocrinol Metab 84:2363–2368
Garnero P, Darte C, Delmas PD (1999) A model to monitor the efficacy of alendronate treatment in women with osteoporosis using a biochemical marker of bone turnover. Bone 24:603–609
Acknowledgments
This study was supported by Wyeth Research, Collegeville, PA, USA, which was acquired by Pfizer Inc in October 2009. Editorial support for the writing of this manuscript was provided by Bo Choi, PhD, and was funded by Wyeth.
Conflicts of interest
Drs. Xu, Tsai, Kim, and Wu do not report conflicts of interest. Drs. Vincendon, Constantine, and Chines were employees of Wyeth, which was acquired by Pfizer Inc in October 2009.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, L., Tsai, KS., Kim, G.S. et al. Efficacy and safety of bazedoxifene in postmenopausal Asian women. Osteoporos Int 22, 559–565 (2011). https://doi.org/10.1007/s00198-010-1259-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-010-1259-5