Abstract
Summary
Dehydroepiandrosterone (DHEA) may be useful in the treatment of postmenopausal osteoporosis (PMO). Our present study has found the preferable stimulatory effect of DHEA on bone, in contrast to the proliferative effects of estradiol (E2) on the endometrium and the uterus, which suggests that DHEA has greater potential clinical value than estrogens in prophylaxis and therapeutics for PMO.
Introduction
A series of findings raise the possibility that DHEA may be useful in the treatment of PMO. Our present study thus aimed at the differential effects of DHEA and E2 on bone and the uterus in ovariectomized mice as well as the involvement of aromatase, ERα, ERβ, and AR in the effects.
Methods
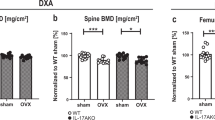
Ovariectomized and sham BALB/c mice were given daily treatment with either DHEA or E2 for three months, respectively. Bone mineral density was determined by DEXA after the last treatment. Mice were necropsied in 3 months after the treatment to analyze the ultrastructure of their femur osteoblasts (OBs) with a transmission electron microscope (TEM); DHEA, DHEA sulfate (DHEAS) and E2 levels were assayed by EIA; production in vitro of E2 in the uterus or tibia was assayed to evaluate the profile of P450arom activity; ERα and ERβ mRNA levels in the uterus and tibia were determined by real-time PCR. The primary murine OBs were treated with DHEA and E2, respectively for 72 h. Real-time polymerase chain reaction (PCR) and western blot were carried out to evaluate aromatase, ERα, ERβ and AR expression in OBs.
Results
Both DHEA and E2 significantly improved BMD and OB ultrastructure; E2 but not DHEA has significantly increased uterus wet weight, endometrium epithelial and gland thickness. Dehydroepiandrosterone not only increased serum, femoral DHEA, DHEAS and E2 concentration, but also increased uterine DHEA and DHEAS other than E2 concentration in site, while E2 only increased serum, uterine and femoral E2 concentration, but failed to alter the concentrations of DHEA and DHEAS. Moreover, DHEA significantly increased tibia P450arom enzyme activity, while E2 increased uterine and tibia aromatase activity. Furthermore, DHEA increased uterine ERβ and ERα, and ERβ transcription in the tibia, while E2 increased ERα transcription in the uterus and tibia. Dehydroepiandrosterone increased aromatase, ERα, ERβ and AR expression in OBs, and increased significantly, but E2 apparently decreased the ratio of ERβ/ERα.
Conclusions
Although both DHEA and E2 augment BMD, the proliferative effects of E2 on the endometrium and uterus reflect the different modes of action on bone and the uterus, indicating that the preferable stimulatory effect of DHEA on bone appears to the more potential clinical values than estrogens in prophylaxis and therapeutics for PMO. But applicability of the findings from rodents in humans needs further study.









Similar content being viewed by others
References
Riggs BL, Khosla S, Melton LJ (2002) Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23:279–302
Vermeulen A (1995) Dehydroepiandrosterone sulfate and aging. Ann NY Acad Sci 774:121–127
Spector TD, Thompson PW, Perry LA, McGarrigle HH, Edwards AC (1991) The relationship between sex steroids and bone mineral content in women soon after the menopause. Clin Endocrinol (Oxf) 34:37–41
Johansson C, Mellstrom D, Milsom I (1993) Reproductive factors as predictors of bone density and fractures in women at the age of 70. Maturitas 17:39–50
Labrie F, Diamond P, Cusan L, Gomez JL, Belanger A, Candas B (1997) Effect of 12-month dehydroepiandrosterone replacement therapy on bone, vagina, and endometrium in postmenopausal women. J Clin Endocrinol Metab 82:3498–3505
Villareal DT (2002) Effects of dehydroepiandrosterone on bone mineral density: what implications for therapy? Treat Endocrinol 1:349–357
Tok EC, Ertunc D, Oz U, Camdeviren H, Ozdemir G, Dilek S (2004) The effect of circulating androgens on bone mineral density in postmenopausal women. Maturitas 48:235–242
Jankowski CM, Gozansky WS, Schwartz RS, Dahl DJ, Kittelson JM, Scott SM, Van Pelt RE, Kohrt WM (2006) Effects of dehydroepiandrosterone replacement therapy on bone mineral density in older adults: a randomized, controlled trial. J Clin Endocrinol Metab 91:2986–2993
Nair KS, Rizza RA, O’Brien P, Dhatariya K, Short KR, Nehra A, Vittone JL, Klee GG, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall DJ, Melton LJ 3rd, Smith GE, Khosla S, Jensen MD (2006) DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med 355:1647–1659
Wang L, Wang YD, Wang WJ, Zhu Y, Li DJ (2007) Dehydroepiandrosterone improves murine osteoblast growth and bone tissue morphometry via mitogen-activated protein kinase signaling pathway independent of either androgen receptor or estrogen receptor. J Mol Endocrinol 38:467–479
Lamberts SW, van den Beld AW, van der Lely AJ (1997) The Endocrinology of Aging. Science 278:419–424
Labrie F, Belanger A, Cusan L, Gomez JL, Candas B (1997) Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated metabolites during aging. J Clin Endocrinol Metab 82:2396–2402
Gen K, Okuzawa K, Kumakura N, Yamaguchi S, Kagawa H (2001) Correlation between messenger RNA expression of cytochrome P450 aromatase and its enzyme activity during oocyte development in the red seabream (Pagrus major). Biol Reprod 65:1186–1194
Bilezikian JP (2002) Sex steroids, mice, and men: when androgens and estrogens get very close to each other. J Bone Miner Res 17:563–566
Couse JF, Korach KS (1999) Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20:358–417
Andersson N, Lindberg MK, Ohlsson C, Andersson K, Ryberg B (2001) Repeated in vivo determinations of bone mineral density during parathyroid hormone treatment in ovariectomized mice. J Endocrinol 170:529–537
Wu X, Jin LP, Yuan MM, Zhu Y, Wang MY, Li DJ (2005) Human first-trimester trophoblast cells recruit CD56brightCD16- NK cells into decidua by way of expressing and secreting of CXCL12/stromal cell-derived factor 1. J Immunol 175:61–68
Lamberts SW (2003) The endocrinology of gonadal involution: menopause and andropause. Ann Endocrinol (Paris) 64:77–81
Cummings SR, Black DM, Nevitt MC, Browner W, Cauley J, Ensrud K, Genant HK, Palermo L, Scott J, Vogt TM (1993) Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet 341:72–75
Melton LJ 3rd, Crowson CS, O’Fallon WM, Wahner HW, Riggs BL (2003) Relative contributions of bone density, bone turnover, and clinical risk factors to long-term fracture prediction. J Bone Miner Res 18:312–318
Kanis JA, Johnell O, Oden A, Johansson H, Eisman JA, Fujiwara S, Kroger H, Honkanen R, Melton LJ 3rd, O’Neill T, Reeve J, Silman A, Tenenhouse A (2006) The use of multiple sites for the diagnosis of osteoporosis. Osteoporos Int 17:527–534
Haden ST, Glowacki J, Hurwitz S, Rosen C, LeBoff MS (2000) Effects of age on serum dehydroepiandrosterone sulfate, IGF-I, and IL-6 levels in women. Calcif Tissue Int 6:414–418
Nawata H, Tanaka S, Tanaka S, Takayanagi R, Sakai Y, Yanase T, Ikuyama S, Haji M (1995) Aromatase in bone cell: association with osteoporosis in postmenopausal women. J Steroid Biochem Mol Biol 53:165–174
Labrie F, Belanger A, Cusan L, Candas B (1997) Physiological changes in dehydroepiandrosterone are not reflected by serum levels of active androgens and estrogens but of their metabolites: intracrinology. J Clin Endocrinol Metab 82:2403–2409
Villareal DT, Holloszy JO, Kohrt WM (2000) Effects of DHEA replacement on bone mineral density and body composition in elderly women and men. Clin Endocrinol (Oxf) 53:561–568
Chow J, Tobias JH, Colston KW, Chambers TJ (1992) Estrogen maintains trabecular bone volume in rats not only by suppression of bone resorption but also by stimulation of bone formation. J Clin Invest 89:74–78
Itani T, Kanai K, Watanabe J, Ogawa R, Kanamura S (1992) Quantitative analysis of rough endoplasmic reticulum in chondrocytes of articular and tracheal cartilage of rabbits following the systemic administration of hydrocortisone. J Anat 181:357–363
Mei Y, Gawai KR, Nie Z, Ramkumar V, Helfert RH (1999) Age-related reductions in the activities of antioxidant enzymes in the rat inferior colliculus. Hear Res 135:169–180
Labrie F, Luu-The V, Labrie C, Simard J (2001) DHEA and its transformation into androgens and estrogens in peripheral target tissues: intracrinology. Front Neuroendocrinol 22:185–212
Nelson JF, Felicio LS, Osterburg HH, Finch CE (1992) Differential contributions of ovarian and extraovarian factors to age-related reductions in plasma estradiol and progesterone during the estrous cycle of C57BL/6J mice. Endocrinology 130:805–810
Walmer DK, Wrona MA, Hughes CL, Nelson KG (1992) Lactoferrin expression in the mouse reproductive tract during the natural estrous cycle: correlation with circulating estradiol and progesterone. Endocrinology 131:1458–1466
Edwards MW, Bain SD, Bailey MC, Lantry MM, Howard GA (1992) 17 beta estradiol stimulation of endosteal bone formation in the ovariectomized mouse: an animal model for the evaluation of bone-targeted estrogens. Bone 13:29–34
Fata JE, Chaudhary V, Khokha R (2001) Cellular turnover in the mammary gland is correlated with systemic levels of progesterone and not 17beta estradiol during the estrous cycle. Biol Reprod 65:680–688
Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M (2001) Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc Natl Acad Sci USA 98:13391–13395
Turner RT, Lifrak ET, Beckner M, Wakley GK, Hannon KS, Parker LN (1990) Dehydroepiandrosterone reduces cancellous bone osteopenia in ovariectomized rats. Am J Physiol 258:E673–E677
Osmanagaoglu MA, Okumus B, Osmanagaoglu T, Bozkaya H (2004) The relationship between serum dehydroepiandrosterone sulfate concentration and bone mineral density, lipids, and hormone replacement therapy in premenopausal and postmenopausal women. J Womens Health (Larchmt) 13:993–999
Lobo RA, Goebelsmann U, Brenner PF, Mishell DR Jr (1982) The effects of estrogen on adrenal androgens in oophorectomized women. Am J Obstet Gynecol 142:471–478
Fonseca E, Basurto L, Velazquez S, Zarate A (2001) Hormone replacement therapy increases ACTH/dehydroepiandrosterone sulfate in menopause. Maturitas 39:57–62
Castelo-Branco C, Martinez de Osaba MJ, Fortuny A, Iglesias X, Gonzalez-Merlo J (1995) Circulating hormone levels in menopausal women receiving different hormone replacement therapy regimens. A comparison. J Reprod Med 40:556–560
Stomati M, Hartmann B, Spinetti A, Mailand D, Rubino S, Albrecht A, Huber J, Petraglia F, Genazzani AR (1996) Effects of hormonal replacement therapy on plasma sex hormone-binding globulin, androgen and insulin-like growth factor-1 levels in postmenopausal women. J Endocrinol Invest 19:535–541
Arlt W, Haas J, Callies F, Reincke M, Hubler D, Oettel M, Ernst M, Schulte HM, Allolio B (1999) Biotransformation of oral dehydroepiandrosterone in elderly men: significant increase in circulating estrogens. J Clin Endocrinol Metab 84:2170–2176
Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD (1994) Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev 15:342–355
Nawata H, Yanase T, Goto K, Okabe T, Nomura M, Ashida K, Watanabe T (2004) Adrenopause. Horm Res 62 Suppl 3:110–114
Purohit A, Flanagan AM, Reed MJ (1992) Estrogen synthesis by osteoblast cell lines. Endocrinology 131:2027–2029
Kim KS, Choi US, Lee SD, Kim KH, Chung KH, Chang YC, Park KK, Lee YC, Kim CH (2005) Effect of bee venom on aromatase expression and activity in leukaemic FLG 29.1 and primary osteoblastic cells. J Ethnopharmacol 99:245–252
Enjuanes A, Garcia-Giralt N, Supervia A, Nogues X, Mellibovsky L, Carbonell J, Grinberg D, Balcells S, Diez-Perez A (2003) Regulation of CYP19 gene expression in primary human osteoblasts: effects of vitamin D and other treatments. Eur J Endocrinol 148:519–526
Meikle AW, Dorchuck RW, Araneo BA, Stringham JD, Evans TG, Spruance SL, Daynes RA (1992) The presence of a dehydroepiandrosterone-specific receptor binding complex in murine T cells. J Steroid Biochem Mol Biol 42:293–304
Okabe T, Haji M, Takayanagi R, Adachi M, Imasaki K, Kurimoto F, Watanabe T, Nawata H (1995) Up-regulation of high-affinity dehydroepiandrosterone binding activity by dehydroepiandrosterone in activated human T lymphocytes. J Clin Endocrinol Metab 80:2993–2996
Kawai S, Yahata N, Nishida S, Nagai K, Mizushima Y (1995) Dehydroepiandrosterone inhibits B16 mouse melanoma cell growth by induction of differentiation. Anticancer Res 15:427–431
Williams MR, Ling S, Dawood T, Hashimura K, Dai A, Li H, Liu JP, Funder JW, Sudhir K, Komesaroff PA (2002) Dehydroepiandrosterone inhibits human vascular smooth muscle cell proliferation independent of ARs and ERs. J Clin Endocrinol Metab 87:176–181
Kalimi M, Regelson W (1988) Physicochemical characterization of [3H] DHEA binding in rat liver. Biochem Biophys Res Commun 156:22–29
Vidal O, Kindblom LG, Ohlsson C (1999) Expression and localization of estrogen receptor-beta in murine and human bone. J Bone Miner Res 14:923–929
De Wilde A, Lieberherr M, Colin C, Pointillart A (2004) A low dose of daidzein acts as an ERbeta-selective agonist in trabecular osteoblasts of young female piglets. J Cell Physiol 200:253–262
Chen FP, Hsu T, Hu CH, Wang WD, Wang KC, Teng LF (2004) Expression of estrogen receptors alpha and beta in human osteoblasts: identification of exon-2 deletion variant of estrogen receptor beta in postmenopausal women. Chang Gung Med J 27:107–115
Bord S, Ireland DC, Beavan SR, Compston JE (2003) The effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblasts. Bone 32:136–141
Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK (2000) Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 407:538–541
Acknowledgments
This work is supported by National Key Research Program of China 2006CB944007 (to D-J Li), National Natural Science Foundation of China No.30472259 (to W-J Wang), the Youth Foundation of Shanghai Hygiene Bureau No.044Y06 (to Y-D Wang), Traditional Chinese Medicine Foundation of Shanghai Public Health Bureau No.2004L020A (to W-J Wang), Shanghai Leading Academic Discipline Project B117 (to D-J Li), Program for Outstanding Medical Academic Leader (to D-J Li).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, L., Wang, YD., Wang, WJ. et al. Differential regulation of dehydroepiandrosterone and estrogen on bone and uterus in ovariectomized mice. Osteoporos Int 20, 79–92 (2009). https://doi.org/10.1007/s00198-008-0631-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-008-0631-1