Abstract
Summary
Most patients who switched to a second bisphosphonate continued their treatment long term, although those who stopped their first drug because of adverse events were likely to discontinue the second drug for the same reason. Switching to another bisphosphonate is a reasonable treatment option for some patients with treatment failure.
Introduction
Patients who experience treatment failure with a bisphosphonate because of adverse events (AEs) or other reasons might receive a second bisphosphonate. However, the frequency and benefits of switching bisphosphonates are unknown.
Methods
We retrospectively evaluated 197 men and 1110 women newly treated with bisphosphonates between 1 January 2000 and 30 June 2005 at our university hospital.
Results
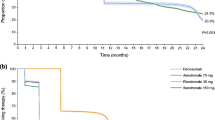
Among the 497 patients who discontinued bisphosphonate treatment, 146 were switched to a second bisphosphonate. The cumulative probabilities of persistence of treatment after 3 years were 45% with the first bisphosphonate and 65% with the second (P = 0.017). Age ≥65 years, switching bisphosphonates because of AEs, and male gender were associated (P < 0.05) with low persistence of treatment with the second bisphosphonate. Discontinuation of the first drug because of AEs was associated with an increased rate of discontinuation of the second drug because of AEs (hazard ratio, 4.2; 95% confidence interval, 2.1–8.4).
Conclusions
Patients who switched bisphosphonates had high rates of persistence of therapy. Those who stopped their first bisphosphonate because of AEs were at risk of discontinuing the second drug for the same reason. Switching to another bisphosphonate is a reasonable treatment option for some patients with treatment failure.

Similar content being viewed by others
References
McClung MR (2000) Bisphosphonates in osteoporosis: recent clinical experience. Expert Opin Pharmacother 1:225–238
Seeman E, Compston J, Adachi J, Brandi ML, Cooper C, Dawson-Hughes B, Jonsson B, Pols H, Cramer JA (2007) Non-compliance: the Achilles’ heel of anti-fracture efficacy. Osteoporos Int 18:711–719
Cramer JA, Gold DT, Silverman SL, Lewiecki EM (2007) A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int 18:1023–1031
Tosteson AN, Grove MR, Hammond CS, Moncur MM, Ray GT, Hebert GM, Pressman AR, Ettinger B (2003) Early discontinuation of treatment for osteoporosis. Am J Med 115:209–216
Ideguchi H, Ohno S, Hattori H, Ishigatsubo Y (2007) Persistence with bisphosphonate therapy including treatment courses with multiple sequential bisphosphonates in the real world. Osteoporos Int 18:1421–1427
Weycker D, Macarios D, Edelsberg J, Oster G (2006) Compliance with drug therapy for postmenopausal osteoporosis. Osteoporos Int 17:1645–1652
Curtis JR, Westfall AO, Allison JJ, Freeman A, Saag KG (2006) Channeling and adherence with alendronate and risedronate among chronic glucocorticoid users. Osteoporos Int 17:1268–1274
Kanis JA, Johnell O, Oden A, Sembo I, Redlund-Johnell I, Dawson A, De Laet C, Jonsson B (2000) Long-term risk of osteoporotic fracture in Malmo. Osteoporos Int 11:669–674
Cranney A, Guyatt G, Griffith L, Wells G, Tugwell P, Rosen C (2002) Meta-analyses of therapies for postmenopausal osteoporosis. IX: Summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocr Rev 23:570–578
Fukunaga M, Kushida K, Kishimoto H, Shiraki M, Taketani Y, Minaguchi H, Inoue T, Morita R, Morii H, Yamamoto K, Ohashi Y, Orimo H (2002) A comparison of the effect of risedronate and etidronate on lumbar bone mineral density in Japanese patients with osteoporosis: a randomized controlled trial. Osteoporos Int 13:971–979
Kushida K, Fukunaga M, Kishimoto H, Shiraki M, Itabashi A, Inoue T, Kaneda K, Morii H, Nawata H, Yamamoto K, Ohashi Y, Orimo H (2004) A comparison of incidences of vertebral fracture in Japanese patients with involutional osteoporosis treated with risedronate and etidronate: a randomized, double-masked trial. J Bone Miner Metab 22:469–478
Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J (2002) Long-term persistence in use of statin therapy in elderly patients. Jama 288:455–461
Miller PD, Woodson G, Licata AA, Ettinger MP, Mako B, Smith ME, Wang L, Yates SJ, Melton ME, Palmisano JJ (2000) Rechallenge of patients who had discontinued alendronate therapy because of upper gastrointestinal symptoms. Clin Ther 22:1433–1442
Maclean C, Newberry S, Maglione M, McMahon M, Ranganath V, Suttorp M, Mojica W, Timmer M, Alexander A, McNamara M, Desai SB, Zhou A, Chen S, Carter J, Tringale C, Valentine D, Johnsen B, Grossman J (2008) Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med 148:197–213
Anastasilakis AD, Goulis DG, Kita M, Avramidis A (2007) Oral bisphosphonate adverse effects in 849 patients with metabolic bone diseases. Hormones (Athens) 6:233–241
Acknowledgements
The authors are greatly indebted to Mr. Tom Kiper (Yokosuka, Japan) for his review and invaluable suggestions in preparing the manuscript.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material:
Fig. S1
Discontinuation of bisphosphonate therapy because of adverse events (AEs) in light of the bisphosphonates used as the first and second drugs. Continuation rates shown are for ETD (dotted line), ALN (solid line), and RIS (dashed line). Use of each drug as the first treatment is shown by fine lines, and as the second treatment by bold lines (PDF 26.3 kb)
Table S1
Numbers of patients who received etidronate (ETD), alendronate (ALN), or risedronate (RIS) as a first or second drug during each year from 1 January 2000 to 30 June 2005 (DOC 31 kb)
Rights and permissions
About this article
Cite this article
Ideguchi, H., Ohno, S., Takase, K. et al. Outcomes after switching from one bisphosphonate to another in 146 patients at a single university hospital. Osteoporos Int 19, 1777–1783 (2008). https://doi.org/10.1007/s00198-008-0618-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-008-0618-y