Abstract
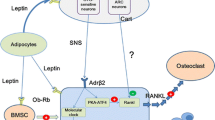
Loss of body weight is associated with bone loss, and body weight gain is associated with increased bone formation. The molecular mechanisms linking body weight, body composition, and bone density are now better understood. Lean mass is likely to have a significant, local effect on bone modeling and remodeling through mechanotransduction pathways. In contrast to the local regulation of bone formation and resorption by muscle-derived stimuli, peripheral body fat appears to influence bone mass via secretion of systemic, endocrine factors that link body weight to bone density even in non-weight bearing regions (e.g., the forearm). The cytokine-like hormone leptin, which is secreted by fat cells, is an important candidate molecule linking changes in body composition with bone formation and bone resorption. Increases in body fat increase leptin levels and stimulate periosteal bone formation through its direct anabolic effects on osteoblasts, and through central (CNS) effects including the stimulation of the GH-IGF-1 axis and suppression of neuropeptide Y, a powerful inhibitor of bone formation. Stimulation of beta2-adrenergic receptors through central (hypothalamic) leptin receptors does, however, increase remodeling of trabecular bone, resulting in a lower cancellous bone volume that may be better adapted to a concomitantly larger cortical bone compartment. These findings suggest that body weight and body fat can regulate bone mass and structure through molecular pathways that are independent of load-bearing. Furthermore, pharmacological manipulation of the signaling pathways activated by leptin may have significant potential for the treatment and prevention of bone loss.

Similar content being viewed by others
References
Rauch F, Bailey D, Baxter-Jones A et al (2004) The ‘muscle-bone unit’ during the pubertal growth spurt. Bone 34:771–775
Greenlund LJ, Nair KS (2003) Sarcopenia-consequences, mechanisms, and potential therapies. Mech Ageing Dev 124:287–299
Huang R, Rubin C, McLeod K (1999) Changes in postural muscle dynamics as a function of age. J Gerontol A Biol Sci Med Sci 54:B352–357
Eisman J (2001) Good, good, good...good vibrations: the best option for better bones? Lancet 358:1924–1925
Schoenau E (2005) From mechanostat theory to development of the “Functional Muscle-Bone Unit”. J Musculoskelet Neuronal Interact 5:232–238
Qin Y, Lam H, Orzechowski L, Xia Y (2005) Bone fluid flow induced by skeletal muscle dynamics and its role in bone adaptation. J Bone Miner Res 20(Suppl 1):F185
Galvard H, Elmstahl S, Elmstahl B et al (1996) Differences in body composition between female geriatric hip fracture patients and healthy controls: body fat is more important as an explanatory factor for the fracture than body weight and lean body mass. Aging Clin Exp Res 8:282–286
Reid I, Ames R, Evans M, Sharpe S, Gamble G, France J, Lim T, Cundy T (1992) Determinants of total body and regional bone mineral density in normal postmenopausal women-a key role for fat mass. J Clin Endocrinol Metab 75:45–51
Fogelholm G, Sievänen H, Kukkonen-Harjula T, Pasanen M (2001) Bone mineral density during reduction, maintenance, and regain of body weight in premenopausal, obese women. Osteoporos Int 12:199–206
Grodin J, Siiteri P, MacDonald P (1973) Source of estrogen production in postmenopausal women. J Clin Endocrinol Metab 36:207–214
Reid I (2002) Relationships among body mass, its components, and bone. Bone 13:547–555
Berner H, Lyngstadaas S, Spahr A et al (2004) Adiponectin and its receptors are expressed in bone-forming cells. Bone 35:842–849
Jürimäe J, Jürimäe T (2007) Adiponectin is a predictor of bone mineral density in middle- aged premenopausal women. Osteoporos Int [Mar 30 ePub ahead of print]
Reid I, Cornish J, Baldock P (2006) Nutrition-related peptides and bone homeostasis. J Bone Miner Res 21:495–500
Hamrick MW (2007) Invited perspective: leptin and bone-a consensus emerging? BoneKey-Osteovision 4:99–107
Flier J (1998) What’s in a name? In search of leptin’s physiologic role. J Clin Endocrinol Metab 83:1407–1413
Soyka L, Grinspoon S, Levitsky L et al (1999) The effects of anorexia nervosa on bone metabolism in female adolescents. J Clin Endocrinol Metab 84:4489–4496
Mantzoros CS (2000) Role of leptin in reproduction. Ann N Y Acad Sci 900:174–183
Frisch R, Revelle R (1970) Height and weight at menarche and a hypothesis of critical body weights and adolescent events. Science 169:397–399
Matkovic V, Ilich J, Skugor M et al (1997) Leptin is inversely related to age at menarche in human females. J Clin Endocrinol Metab 82:3239–3245
Rosenthal D, Mayo-Smith W, Hayes C et al (1989) Age and bone mass in premenopausal women. J Bone Miner Res 4:533–538
Bonjour J-P, Chevalley T (2007) Pubertal timing, peak bone mass and fragility fracture risk. BoneKEy-Osteovision 4:30–48
Clark EM, Ness AR, Tobias JH (2006) Adipose tissue stimulates bone growth in prepubertal children. J Clin Endocrinol Metab 91:2534–2541
Chehab F, Lim ME, Lu R (1996) Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet 12:318–320
Carro E, Senaris R, Considine R, Casanueva F, Dieguez C (1997) Regulation of in vivo growth hormone secretion by leptin. Endocrinology 138:2203–2206
Gat-Yablonski G, Ben-Ari T, Shaif B et al (2004) Leptin reverses the inhibitory effect of caloric restriction on longitudinal growth. Endocrinology 145:343–350
Welt C, Chan J, Bullen J et al (2004) Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med 351:987–997
Yamauchi M, Sugimoto T, Yamaguchi T, Nakaoka D, Kanzawa M, Yano S, Ozuru R, Sugishita T, Chihara K (2001) Plasma leptin concentrations are associated with bone mineral density and the presence of vertebral fractures in postmenopausal women. Clin Endocrinol 55:341–347
Thomas T, Burguera B, Melton L et al (2001) Role of serum leptin, insulin, and estrogen levels as potential mediators of the relationship between fat mass and bone mineral density in men versus women. Bone 29:114–120
Blain H, Vuillemin A, Guillemin F et al (2002) Serum leptin level is a predictor of bone mineral density in postmenopausal women. J Clin Endocrinol Metab 87:1030–1035
Weiss L, Barrett-Connor E, von Muhlen D, Clark P (2006) Leptin predicts BMD and one resorption in older women but not older men: the Rancho-Bernardo study. J Bone Miner Res 21:758–764
Lorentzon M, Landin K, Mellström D, Ohlsson C (2006) Leptin is a negative independent predictor of areal BMD and cortical bone size in young adult Swedish men. J Bone Miner Res 21:1871–1878
Martin A, David V, Malaval L et al (2007) Opposite effects of leptin on bone metabolism: a dose-dependent balance related to energy intake and insulin-like growth factor-1 pathway. Endocrinology 148:3419–3425
Considine RV, Considine EL, Williams CJ et al (1996) The hypothalamic leptin receptor in humans: identification of incidental sequence polymorphisms and absence of the db/db mouse and fa/fa rat mutations. Diabetes 45:992–994
Matsuoka N, Ogawa Y, Hosoda K et al (1997) Human leptin receptor gene in obese Japanese subjects: evidence against either obesity-causing mutations or association of sequence variants with obesity. Diabetologia 40:1204–1210
Chung WK, Power-Kehoe L, Chua M et al (1997) Exonic and intronic sequence variation in the human leptin receptor gene (LEPR). Diabetes 46:1509–1511
Quinton ND, Lee AJ, Ross RJ, Eastell R, Blakemore AI (2001) A single nucleotide polymorphism (SNP) in the leptin receptor is associated with BMI, fat mass and leptin levels in postmenopausal Caucasian women. Hum Genet 108:233–236
Chagnon YC, Wilmore JH, Borecki IB et al (2000) Associations between the leptin receptor gene and adiposity in middle-aged Caucasian males from the HERITAGE family study. J Clin Endocrinol Metab 85:29–34
Fairbrother UL, Tanko LB, Walley AJ et al (2007) Leptin receptor genotype at Gln223Arg is associated with body composition, BMD, and vertebral fracture in postmenopausal Danish women. J Bone Miner Res 22:544–550
Koh JM, Kim DJ, Hong JS et al (2002) Estrogen receptor alpha gene polymorphisms Pvu II and Xba I influence association between leptin receptor gene polymorphism (Gln223Arg) and bone mineral density in young men. Eur J Endocrinol 147:777–783
Crabbe P, Goemaere S, Zmierczak H et al (2006) Are serum leptin and the Gln223Arg polymorphism of the leptin receptor determinants of bone homeostasis in elderly men? Eur J Endocrinol 154:707–714
Richert L, Chevalley T, Manen D et al (2007) Bone mass in prepubertal boys is associated with a Gln223Arg amino acid substitution in the leptin receptor. J Clin Endocrinol Metab
Thomas T (2004) The complex effects of leptin on bone metabolism through multiple pathways. Curr Opin Pharmacol 4:295–300
Holloway WR, Collier FM, Aitken CJ et al (2002) Leptin inhibits osteoclast generation. J Bone Miner Res 17:200–209
Burguera B, Hofbauer L, Thomas T et al (2001) Leptin reduces ovariectomy-induced bone loss in rats. Endocrinology 142:3546–3553
Thomas T, Gori F, Khosla S et al (1999) Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology 140:1630–1638
Hess R, Pino A, Rios S, Fernandez M, Rodriguez J (2004) High affinity leptin receptors are present in human mesenchymal stem cells (MSCs) derived from control and osteoporotic donors. J Cellular Biochem 94:50–57
Laharrague P, Larrouy D, Fontanilles AM (1998) High expression of leptin by human bone marrow adipocytes in primary culture. FASEB J 12:747–752
Kim G, Hong J, Kim S et al (2003) Leptin induces apoptosis via ERK/cPLA2/Cytochrome c pathway in human bone marrow stromal cells. J Biol Chem 278:21920–21929
Hamrick M, Della-Fera MA, Hartzell D, Choi Y-H, Baile CA (2007) Intrahypothalamic injections of leptin increase adipocyte apoptosis in peripheral fat pad and in bone marrow. Cell Tissue Res 327:133–141
Meunier P, Aaron J, Edouard C, Vignon G (1971) Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. Clin Orthop Rel Res 80:147–154
Kajkenova O, Lecka-Czernik F, Gubrij I et al (1997) Increased adipogenesis and myelopoiesis in the bone marrow of SAMP6, a murine model of defective osteoblastogenesis and low turnover osteopenia. J Bone Miner Res 12:1772–1779
Justesen J, Stenderup K, Ebbesen E et al (2001) Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2:165–171
Verma S, Rajaratnam J, Denton J et al (2002) Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol 55:693–698
Lazarenko O, Rzonca S, Hogue W et al (2007) Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology 148:2669–2680
Schwartz A, Sellmeyer D, Vittinghoff D et al (2006) Thiazolidinedione use and bone loss in older diabetic adults. J Clin Endocrinol Metab 91:3349–3354
Elmquist JK, Maratos-Flier E, Saper CB et al (1998) Unraveling the central nervous system pathways underlying responses to leptin. Nat Neurosci 1:445–450
Ducy P, Amling M, Takeda S, Priemel M, Schilling A, Beil F, Shen J, Vinson C, Rueger J, Karsenty G (2000) Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100:197–207
Elefteriou F, Ahn JD, Takeda S et al (2005) Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434:514–520
Hamrick MW, Pennington C, Newton D, Xie D, Isales C (2004) Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone 34:376–383
Baldock PA, Allison S, McDonald MM et al (2006) Hypothalamic regulation of cortical bone mass: opposing activity of Y2 receptor and leptin pathways. J Bone Miner Res 21:1600–1607
Kellenberger S, Muller K, Richener H, Bilbe G (1998) Formoterol and isoproterenol induce c-fos gene expression in osteoblast-like cells by activating beta2-adrenergic receptors. Bone 22:471–478
Moore RE, Smith CK, II, Bailey CS, Voelkel EF, Tashjian AH, Jr (1993) Characterization of beta-adrenergic receptors on rat and human osteoblast-like cells and demonstration that beta-receptor agonists can stimulate bone resorption in organ culture. Bone Miner 23:301–315
Suzuki A, Guicheux J, Palmer G et al (2002) Evidence for a role of p38 MAP kinase in expression of alkaline phosphatase during osteoblastic cell differentiation. Bone 30:91–98
Suzuki A, Palmer G, Bonjour JP, Caverzasio J (1998) Catecholamines stimulate the proliferation and alkaline phosphatase activity of MC3T3-E1 osteoblast-like cells. Bone 23:197–203
Takeda S, Elefteriou F, Levasseur R et al (2002) Leptin regulates bone formation via the sympathetic nervous system. Cell 111:305–317
Togari A, Arai M, Mizutani S, Mizutani S, Koshihara Y, Nagatsu T (1997) Expression of mRNAs for neuropeptide receptors and beta-adrenergic receptors in human osteoblasts and human osteogenic sarcoma cells. Neurosci Lett 233:125–128
Arai M, Nagasawa T, Koshihara Y, Yamamoto S, Togari A (2003) Effects of beta-adrenergic agonists on bone-resorbing activity in human osteoclast-like cells. Biochim Biophys Acta 1640:137–142
Togari A, Mogi M, Arai M, Yamamoto S, Koshihara Y (2000) Expression of mRNA for axon guidance molecules, such as semaphorin-III, netrins and neurotrophins, in human osteoblasts and osteoclasts. Brain Res 878:204–209
Takeuchi T, Tsuboi T, Arai M, Togari A (2001) Adrenergic stimulation of osteoclastogenesis mediated by expression of osteoclast differentiation factor in MC3T3-E1 osteoblast-like cells. Biochem Pharmacol 61:579–586
Glatt V, Canalis E, Stadmeyer L, M Bouxsein (2007) Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res 22:1197–1207
Cao J, Venton L, Sakata T, Halloran BP (2003) Expression of RANKL and OPG correlates with age-related bone loss in male C57BL/6 mice. J Bone Miner Res 18:270–277
Pierroz D, Muzzin P, Glatt V et al (2004) β1β2-adrenergic receptor ko mice have decreased total body and cortical bone mass despite increased trabecular bone number. J Bone Miner Res 19(supp 1):1121
Fu L, Patel M, Bradley A et al (2005) The molecular clock mediates leptin-regulated bone formation. Cell 122:803–815
Turek F, Joshu C, Kohsaka A et al (2005) Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308:1043–1045
Gomez-Abellan P, Hernandez-Morante, Lujan J et al (2007) Clock genes are implicated in the human metabolic syndrome. Int J Obesity 1–8
Pierroz D, Bouxsein M, Cavat F et al (2006) Synergistic effects of adrenergic blockade and intermittent parathyroid hormone on bone in ovariectomized mice. Bone 39:260–267
Bonnet N, Beaupied H, Vico L et al (2007) Combined effects of exercise and propranolol on bone tissue in ovariectomized rats. J Bone Miner Res 22:578–588
Bonnet N, Laroche N, Vico L et al (2006) Dose effects of propranolol on cancellous and cortical bone in ovariectomized adult rats. J Pharmacol Exp Ther 318:1118–1127
Pierroz D, Bouxsein ML, Muzzin P et al (2005) Bone loss following ovariectomy is maintained in absence of adrenergic receptor ß1 and ß2 signaling. J Bone Miner Res 20(Suppl.1):S277
Dhillon H, Glatt V, Ferrari S, Bouxsein ML (2004) β-Adrenergic receptor KO mice have increased bone mass and strength but are not protected from ovariectomy-induced bone loss. J Bone Miner Res 19(Suppl.1):S32
Marenzana M, De Souza RL, Chenu C (2007) Blockade of beta-adrenergic signaling does not influence the bone mechano-adaptive response in mice. Bone 41:206–215
Zeman RJ, Hirschman A, Hirschman ML et al (1991) Clenbuterol, a beta 2-receptor agonist, reduces net bone loss in denervated hindlimbs. Am J Physiol 261(2 Pt 1):E285–E289
Baker JG, Hall IP, Hill SJ (2003) Agonist and inverse agonist actions of beta-blockers at the human beta 2-adrenoceptor provide evidence for agonist-directed signaling. Mol Pharmacol 64:1357–1369
Pierroz D, Baldock PA, Bouxsein ML, Ferrari S (2006) Low cortical bone mass in mice lacking beta 1 and beta 2 adrenergic receptors is associated with low bone formation and circulating IGF-1. J Bone Min Res 21(Suppl. 1):S26
Levasseur R, Dargent-Molina P, Sabatier JP et al (2005) Beta-blocker use, bone mineral density, and fracture risk in older women: results from the Epidemiologie de l’Osteoporose prospective study. J Am Geriatr Soc 53:550–552
Pasco JA, Henry MJ, Sanders KM et al (2004) Beta-adrenergic blockers reduce the risk of fracture partly by increasing bone mineral density: Geelong osteoporosis study. J Bone Miner Res 19:19–24
Reid IR, Gamble GD, Grey AB, et al (2005) Beta-Blocker use, BMD, and fractures in the study of osteoporotic fractures. J Bone Miner Res 20:613–618
Reid IR, Lucas J, Wattie D et al (2005) Effects of a beta-blocker on bone turnover in normal postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab 90:5212–5216
Rejnmark L, Vestergaard P, Kassem M et al (2004) Fracture risk in perimenopausal women treated with beta-blockers. Calcif Tissue Int 75:365–372
Rejnmark L, Vestergaard P, Mosekilde L (2006) Treatment with beta-blockers, ACE inhibitors, and calcium-channel blockers is associated with a reduced fracture risk: a nationwide case-control study. J Hypertens 24:581–589
Schlienger RG, Kraenzlin ME, Jick SS et al (2004) Use of beta-blockers and risk fractures. JAMA 292:1326–1332
Khosla S (2002) Leptin-central or peripheral to the regulation of bone metabolism? Endocrinology 143:4161–4164
Hamrick MW (2004) Leptin, bone mass, & the thrifty phenotype. J Bone Miner Res 19:1607–1611
Baldock P, Allison S, McDonald M et al (2006) Hypothalamic regulation of cortical bone mass: opposing activity of Y2 receptor and leptin pathways. J Bone Miner Res 21:1600–1607
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamrick, M.W., Ferrari, S.L. Leptin and the sympathetic connection of fat to bone. Osteoporos Int 19, 905–912 (2008). https://doi.org/10.1007/s00198-007-0487-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-007-0487-9