Abstract
Introduction
Spinal cord injury (SCI) causes a significant amount of bone loss in the sublesional area in animals and humans, and this type of bone loss is different from other forms of osteoporosis such as disuse osteoporosis and postmenopausal osteoporosis. However, no data is available on the cellular and molecular changes of osteoblastogenesis and osteoclastogenesis during SCI-induced bone loss.
Methods
SCI and SHAM rats were used in this study to investigate osteoblastogenesis and osteoclastogenesis in bone-marrow culture. We also measured bone mass and bone histomorphometry, as well as the expression of alkaline phosphatase (ALP), core binding factor α1 (Cbfa-1), osterix, receptor activator of NF-κB ligand (RANKL) and osteoprotegerin (OPG) in osteoblast-like cells in bone-marrow culture obtained from SCI and SHAM rats.
Results
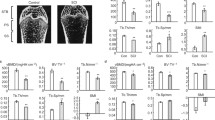
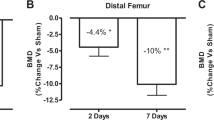
Bone mineral density (BMD) measurement showed serious bone loss in the tibial ephiphyses and metaphyses of SCI rats compared with SHAM rats. In addition, bone histomorphometry analysis of the tibial metaphyses of SCI rats demonstrated that bone microarchitecture in SCI rats deteriorated further than in SHAM rats, and increased eroded surfaces and bone formation rates were observed in SCI rats. The number of osteoclasts that developed from bone marrow of SCI rats at equal density was significantly increased compared with SHAM rats, and the area of the resorption pits formed in the bone marrow culture from SCI rats was significantly greater than SHAM rats, whereas the number of CFU-F and CFU-OB was similar in both groups. RANKL mRNA and protein levels in osteoblast-like cells in culture obtained from SCI rats were significantly higher than those from the SHAM rats, whereas OPG levels decreased slightly. The ratios of RANKL to OPG expression in SCI rats were significantly higher than those in SHAM rats. However, osteogenic gene profiling of Cbfa-1, ALP and osterix in SCI rats remained similar with SHAM rats.
Conclusion
These changes favor increased osteoclast activity over osteoblast activity, and may explain, in part, the imbalance in bone formation and resorption following SCI.




Similar content being viewed by others
References
Hill EL, Martin RB, Gunther E, Morey-Holton E, Holets VR (1993) Changes in bone in a model of spinal cord injury. J Orthop Res 11:537–547
Demirel G, Yilmaz H, Paker N, Onel S (1998) Osteoporosis after spinal cord injury. Spinal Cord 36:822–825
Szollar SM, Martin EM, Parthemore JG, Sartoris DJ, Deftos LJ (1997) Demineralization in tetraplegic and paraplegic man over time. Spinal Cord 35:223–228
Szollar SM, Martin EM, Parthemore JG, Sartoris DJ, Deftos LJ (1997) Densitometric patterns of spinal cord injury associated bone loss. Spinal Cord 35:374–382
Chow YW, Inman C, Pollintine P, Sharp CA, Haddaway MJ, el Masry W, Davie MW (1996) Ultrasound bone densitometry and dual energy X-ray absorptiometry in patients with spinal cord injury: a cross-sectional study. Spinal Cord 34:736–741
Wilmet E, Ismail AA, Heilporn A, Welraeds D, Bergmann P (1995) Longitudinal study of the bone mineral content and of soft tissue composition after spinal cord section. Paraplegia 33:674–677
Garland DE, Stewart CA, Adkins RH, Hu SS, Rosen C, Liotta FJ, Weinstein DA (1992) Osteoporosis after spinal cord injury. J Orthop Res 10:371–378
Kiratli BJ, Smith AE, Nauenberg T, Kallfelz CF, Perkash I (2000) Bone mineral and geometric changes through the femur with immobilization due to spinal cord injury. J Rehabil Res Dev 37:225–233
Takata S, Yasui N (2001) Disuse osteoporosis. J Med Invest 48:147–156
Uebelhart D, Demiaux-Domenech B, Roth M, Chantraine A (1995) Bone metabolism in spinal cord injured individuals and in others who have prolonged immobilisation. A review. Paraplegia 33:669–673
Dauty M, Perrouin Verbe B, Maugars Y, Dubois C, Mathe JF (2000) Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone 27:305–309
Biering-Sorensen F, Bohr HH, Schaadt OP (1990) Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur J Clin Invest 20:330–335
Finsen V, Indredavik B, Fougner KJ (1992) Bone mineral and hormone status in paraplegics. Paraplegia 30:343–347
Leblanc AD, Schneider VS, Evans HJ, Engelbretson DA, Krebs JM (1990) Bone mineral loss and recovery after 17 weeks of bed rest. J Bone Miner Res 5:843–850
Maimoun L, Couret I, Micallef JP, Peruchon E, Mariano-Goulart D, Rossi M, Leroux JL, Ohanna F (2002) Use of bone biochemical markers with dual-energy X-ray absorptiometry for early determination of bone loss in persons with spinal cord injury. Metabolism 51:958–963
Roberts D, Lee W, Cuneo RC, Wittmann J, Ward G, Flatman R, McWhinney B, Hickman PE (1998) Longitudinal study of bone turnover after acute spinal cord injury. J Clin Endocrinol Metab 83:415–422
Smith SM, Nillen JL, Leblanc A, Lipton A, Demers LM, Lane HW, Leach CS (1998) Collagen cross-link excretion during space flight and bed rest. J Clin Endocrinol Metab 83:3584–3591
Lueken SA, Arnaud SB, Taylor AK, Baylink DJ (1993) Changes in markers of bone formation and resorption in a bed rest model of weightlessness. J Bone Miner Res 8:1433–1438
Inoue M, Tanaka H, Moriwake T, Oka M, Sekiguchi C, Seino Y (2000) Altered biochemical markers of bone turnover in humans during 120 days of bed rest. Bone 26:281–286
Jones LM, Legge M, Goulding A (2002) Intensive exercise may preserve bone mass of the upper limbs in spinal cord injured males but does not retard demineralisation of the lower body. Spinal Cord 40:230–235
Parfitt AM (1994) Osteonal and hemi-osteonal remodeling: the spatial and temporal framework for signal traffic in adult human bone. J Cell Biochem 55:273–286
Manolagas SC (2000) Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21:115–137
Demulder A, Guns M, Ismail A, Wilmet E, Fondu P, Bergmann P (1998) Increased osteoclast-like cells formation in long-term bone marrow cultures from patients with a spinal cord injury. Calcif Tissue Int 63:396–400
Owen ME, Cave J, Joyner CJ (1987) Clonal analysis in vitro of osteogenic differentiation of marrow CFU-F. J Cell Sci 87(Pt 5):731–738
Pilonchery G, Minaire P, Milan JJ, Revol A (1983) Urinary elimination of glycosaminoglycans during the immobilization osteoporosis of spinal cord injury patients. Clin Orthop 174:230–235
Roodman GD, Ibbotson KJ, MacDonald BR, Kuehl TJ, Mundy GR (1985) 1,25-Dihydroxyvitamin D3 causes formation of multinucleated cells with several osteoclast characteristics in cultures of primate marrow. Proc Natl Acad Sci USA 82:8213–8217
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423:337–342
Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G (1997) Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89:747–754
Karsenty G (2001) Minireview: transcriptional control of osteoblast differentiation. Endocrinology 142:2731–2733
Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T (1997) Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89:755–764
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89:309–319
Jimi E, Akiyama S, Tsurukai T, Okahashi N, Kobayashi K, Udagawa N, Nishihara T, Takahashi N, Suda T (1999) Osteoclast differentiation factor acts as a multifunctional regulator in murine osteoclast differentiation and function. J Immunol 163:434–442
Burgess TL, Qian Y, Kaufman S, Ring BD, Van G, Capparelli C, Kelley M, Hsu H, Boyle WJ, Dunstan CR, Hu S, Lacey DL (1999) The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. J Cell Biol 145:527–538
Lacey DL, Tan HL, Lu J, Kaufman S, Van G, Qiu W, Rattan A, Scully S, Fletcher F, Juan T, Kelley M, Burgess TL, Boyle WJ, Polverino AJ (2000) Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. Am J Pathol 157:435–448
O’Brien EA, Williams JH, Marshall MJ (2000) Osteoprotegerin ligand regulates osteoclast adherence to the bone surface in mouse calvaria. Biochem Biophys Res Commun 274:281–290
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610
Yao GQ, Sun BH, Weir EC, Insogna KL (2002) A role for cell-surface CSF-1 in osteoblast-mediated osteoclastogenesis. Calcif Tissue Int 70:339–346
Murrills RJ, Dempster DW (1990) The effects of stimulators of intracellular cyclic AMP on rat and chick osteoclasts in vitro: validation of a simplified light microscope assay of bone resorption. Bone 11:333–344
Chantraine A, Nusgens B, Lapiere CM (1986) Bone remodeling during the development of osteoporosis in paraplegia. Calcif Tissue Int 38:323–327
Chantraine A, van Ouwenaller C, Hachen HJ, Schinas P (1979) Intra-medullary pressure and intra-osseous phlebography in paraplegia. Paraplegia 17:391–399
Minaire P, Edouard C, Arlot M, Meunier PJ (1984) Marrow changes in paraplegic patients. Calcif Tissue Int 36:338–340
Chantraine A (1978) Actual concept of osteoporosis in paraplegia. Paraplegia 16:51–58
Bergmann P, Heilporn A, Schoutens A, Paternot J, Tricot A (1977) Longitudinal study of calcium and bone metabolism in paraplegic patients. Paraplegia 15:147–159
Maynard FM (1986) Immobilization hypercalcemia following spinal cord injury. Arch Phys Med Rehabil 67:41–44
Mechanick JI, Pomerantz F, Flanagan S, Stein A, Gordon WA, Ragnarsson KT (1997) Parathyroid hormone suppression in spinal cord injury patients is associated with the degree of neurologic impairment and not the level of injury. Arch Phys Med Rehabil 78:692–696
Bauman WA, Zhong YG, Schwartz E (1995) Vitamin D deficiency in veterans with chronic spinal cord injury. Metabolism 44:1612–1616
Vaziri ND, Pandian MR, Segal JL, Winer RL, Eltorai I, Brunnemann S (1994) Vitamin D, parathormone, and calcitonin profiles in persons with long-standing spinal cord injury. Arch Phys Med Rehabil 75:766–769
Hjeltnes N, De Groot P, Birkeland KI, Falch JA, Iversen PO (2005) Tetraplegic subjects have hyperleptinaemia with marked circadian variation. Clin Endocrinol (Oxf) 62:223–227
Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei MJ, Pomonis JD, Keyser CP, Clohisy DR, Adams DJ, O’Leary P, Mantyh PW (2002) Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience 113:155–166
Serre CM, Farlay D, Delmas PD, Chenu C (1999) Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone 25:623–629
Hara-Irie F, Amizuka N, Ozawa H (1996) Immunohistochemical and ultrastructural localization of CGRP-positive nerve fibers at the epiphyseal trabecules facing the growth plate of rat femurs. Bone 18:29–39
Strange-Vognsen HH, Laursen H (1997) Nerves in human epiphyseal uncalcified cartilage. J Pediatr Orthop B 6:56–58
Bliziotes MM, Eshleman AJ, Zhang XW, Wiren KM (2001) Neurotransmitter action in osteoblasts: expression of a functional system for serotonin receptor activation and reuptake. Bone 29:477–486
Itzstein C, Espinosa L, Delmas PD, Chenu C (2000) Specific antagonists of NMDA receptors prevent osteoclast sealing zone formation required for bone resorption. Biochem Biophys Res Commun 268:201–209
Lundberg P, Lie A, Bjurholm A, Lehenkari PP, Horton MA, Lerner UH, Ransjo M (2000) Vasoactive intestinal peptide regulates osteoclast activity via specific binding sites on both osteoclasts and osteoblasts. Bone 27:803–810
Suzuki A, Palmer G, Bonjour JP, Caverzasio J (1998) Catecholamines stimulate the proliferation and alkaline phosphatase activity of MC3T3-E1 osteoblast-like cells. Bone 23:197–203
Burt-Pichat B, Lafage-Proust MH, Duboeuf F, Laroche N, Itzstein C, Vico L, Delmas PD, Chenu C (2005) Dramatic decrease of innervation density in bone after ovariectomy. Endocrinology 146:503–510
Imai S, Tokunaga Y, Konttinen YT, Maeda T, Hukuda S, Santavirta S (1997) Ultrastructure of the synovial sensory peptidergic fibers is distinctively altered in different phases of adjuvant induced arthritis in rats: ultramorphological characterization combined with morphometric and immunohistochemical study for substance P, calcitonin gene related peptide, and protein gene product 9.5. J Rheumatol 24:2177–2187
Slade JM, Bickel CS, Modlesky CM, Majumdar S, Dudley GA (2005) Trabecular bone is more deteriorated in spinal cord injured versus estrogen-free postmenopausal women. Osteoporos Int 16:263–272
Modlesky CM, Majumdar S, Narasimhan A, Dudley GA (2004) Trabecular bone microarchitecture is deteriorated in men with spinal cord injury. J Bone Miner Res 19:48–55
Egrise D, Holy X, Hinsenkamp M, Begot L, Schoutens A, Bergmann P, Zerath E (2003) Protracted systemic changes in bone biology after segmented unloading in the rat. Calcif Tissue Int 73:56–65
Halloran BP, Bikle DD, Wronski TJ, Globus RK, Levens MJ, Morey-Holton E (1986) The role of 1,25-dihydroxyvitamin D in the inhibition of bone formation induced by skeletal unloading. Endocrinology 118:948–954
Fazzalari NL, Kuliwaba JS, Atkins GJ, Forwood MR, Findlay DM (2001) The ratio of messenger RNA levels of receptor activator of nuclear factor kappaB ligand to osteoprotegerin correlates with bone remodeling indices in normal human cancellous bone but not in osteoarthritis. J Bone Miner Res 16:1015–1027
Morton A (2003) Endocrine profiles and semen quality in spinal cord injured men. Clin Endocrinol (Oxf) 59:534–535
Tsitouras PD, Zhong YG, Spungen AM, Bauman WA (1995) Serum testosterone and growth hormone/insulin-like growth factor-I in adults with spinal cord injury. Horm Metab Res 27:287–292
Huang TS, Wang YH, Lien IN (1995) Suppression of the hypothalamus–pituitary somatotrope axis in men with spinal cord injuries. Metabolism 44:1116–1120
Huang TS, Wang YH, Chiang HS, Lien YN (1993) Pituitary–testicular and pituitary–thyroid axes in spinal cord-injured males. Metabolism 42:516–521
Huang TS, Wang YH, Lai JS, Chang CC, Lien IN (1996) The hypothalamus-pituitary-ovary and hypothalamus-pituitary-thyroid axes in spinal cord-injured women. Metabolism 45:718–722
Bjurholm A, Kreicbergs A, Brodin E, Schultzberg M (1988) Substance P- and CGRP-immunoreactive nerves in bone. Peptides 9:165–171
Hill EL, Elde R (1988) Calcitonin gene-related peptide-immunoreactive nerve fibers in mandibular periosteum of rat: evidence for primary afferent origin. Neurosci Lett 85:172–178
Hukkanen M, Konttinen YT, Rees RG, Gibson SJ, Santavirta S, Polak JM (1992) Innervation of bone from healthy and arthritic rats by substance P and calcitonin gene related peptide containing sensory fibers. J Rheumatol 19:1252–1259
Ishizuka K, Hirukawa K, Nakamura H, Togari A (2005) Inhibitory effect of CGRP on osteoclast formation by mouse bone marrow cells treated with isoproterenol. Neurosci Lett 379:47–51
Mukohyama H, Ransjo M, Taniguchi H, Ohyama T, Lerner UH (2000) The inhibitory effects of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide on osteoclast formation are associated with upregulation of osteoprotegerin and downregulation of RANKL and RANK. Biochem Biophys Res Commun 271:158–163
Hofbauer LC, Kuhne CA, Viereck V (2004) The OPG/RANKL/RANK system in metabolic bone diseases. J Musculoskelet Neuronal Interact 4:268–275
Maimoun L, Couret I, Mariano-Goulart D, Dupuy AM, Micallef JP, Peruchon E, Ohanna F, Cristol JP, Rossi M, Leroux JL (2005) Changes in osteoprotegerin/RANKL system, bone mineral density, and bone biochemicals markers in patients with recent spinal cord injury. Calcif Tissue Int 76:404–411
Yano K, Tsuda E, Washida N, Kobayashi F, Goto M, Harada A, Ikeda K, Higashio K, Yamada Y (1999) Immunological characterization of circulating osteoprotegerin/osteoclastogenesis inhibitory factor: increased serum concentrations in postmenopausal women with osteoporosis. J Bone Miner Res 14:518–527
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, SD., Jiang, LS. & Dai, LY. Effects of spinal cord injury on osteoblastogenesis, osteoclastogenesis and gene expression profiling in osteoblasts in young rats. Osteoporos Int 18, 339–349 (2007). https://doi.org/10.1007/s00198-006-0229-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-006-0229-4