Abstract
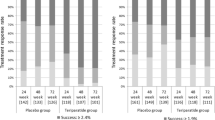
It is commonly believed that the response to treatment in patients on alendronate is proportional to the increase in bone mineral density (BMD), and that those who lose BMD during treatment might not respond to treatment. In the Fracture Intervention Trial 6,459 women were randomly assigned to treatment with alendronate or placebo; BMD was measured annually, and new spine fractures were assessed by lateral spine films, taken at baseline and end of follow-up. Among subjects who took at least 70% of the study drug (5,220 women), we compared reductions in risk of spine fractures at end of follow-up (3 or 4 years) within various levels of change in total hip and spine BMD after 1 and 2 years of treatment, after adjustment for differences in characteristics between the treatment and control groups. Women “losing” BMD at the lumbar spine (0% to 4%) while on alendronate had a reduction of 60% in vertebral fracture risk [OR=0.40 (0.16, 0.99)] compared to their counterparts in the placebo group. The few women that lost more than 4% did not have a significant benefit [OR=0.15 (0.02, 1.29)]. Those who “gained” BMD (0% to 4%) during treatment had a reduction in risk of 51% [OR=0.49 (0.30, 0.78)]. Similarly, women who “lost” total hip BMD (0% to 4%) during the first year on alendronate had a 53% decreased risk of vertebral fracture compared to their controls taking placebo [OR=0.47 (0.27, 0.81)], whereas those “gaining” BMD (0% to 4%) had a comparable risk reduction [OR=0.49 (0.34, 0.71)]. This was not observed for the few women who lost more than 4% [OR=0.61 (0.11, 3.45)]. Patients who lost BMD at both the hip and spine were not protected by alendronate. Among patients who adhere to treatment with alendronate, even those who lose BMD benefit from a substantial reduction in risk of vertebral fracture. So, the reduction in bone turnover induced by alendronate might be more important than BMD changes. The few women who lose the most BMD (more than 4% per year) might not benefit from the treatment.






Similar content being viewed by others
References
Miller PD (2001) New possibilities for diagnosis and treatment of osteoporosis. Int J Fertil Women Med 31: 215–221
Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, et al (1995) Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med 333:1437–1443
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 348:1535–1541
Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, et al (1998) Effect of alendronate on risk of fracture in women with low bone mineral density but without vertebral fractures. JAMA 280:2077–2082
Hochberg MC, Ross PD, Black D, Cummings SR, Genant HK, Nevitt MC, et al (1999) Larger increases in bone mineral density during alendronate therapy are associated with a lower risk of new vertebral fractures in women with postmenopausal osteoporosis. Arthritis Rheum 42:1246–1254
Black DM, Reiss TF, Nevitt MC, Cauley J, Karpf D, Cummings SR (1993) Design of the Fracture Intervention Trial. Osteoporos Int 3 [Suppl 3]:S29–S39
National Osteoporosis Foundation Working Group on Vertebral Fracture (1995) J Bone Miner Res 10:518–523
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semi-quantitative technique. J Bone Miner Res 8:1137–1148
Cummings SR, Palermo L, Browner W, Marcus R, Wallace R, Pearson J, et al (2000) Monitoring osteoporosis therapy with bone densitometry. Misleading changes and regression to the mean. JAMA 283:1318–1321
Cummings SR, Black DM, Pearson J, Karpf DB, Harris F, Genant HK, Ensrud K, LaCroix A (2002) Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med 112:281–289
Parfitt AM (1987) Trabecular bone architecture in the pathogenesis and prevention of fracture. Am J Med 82:68–72
Bauer DC, Black DM, Garnero P, Hochberg M, Ott S, Orloff J, Thompson DE, Ewing SK, Delmas PD (2004) Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the Fracture Intervention Trial. J Bone Miner Res 19:1250–1258
Recker RR (1993) Architecture and vertebral fracture. Calcif Tissue Int 53 [Suppl 1]:S139–S142
Mosekilde L (1993) Vertebral structure and strength in vivo and in vitro. Calcif Tissue Int 53 [Suppl 1]:S121–S125
Delmas PD (2000) How does antiresorptive therapy decrease the risk of fracture in women with osteoporosis? Bone; 27:1–3
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper was written for the Fracture Intervention Trial Research Group
Presented in part at the 22nd Annual Meeting of the American Society for Bone and Mineral Research, Toronto, Canada, 2000
Rights and permissions
About this article
Cite this article
Chapurlat, R.D., Palermo, L., Ramsay, P. et al. Risk of fracture among women who lose bone density during treatment with alendronate. The Fracture Intervention Trial. Osteoporos Int 16, 842–848 (2005). https://doi.org/10.1007/s00198-004-1770-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-004-1770-7