Abstract
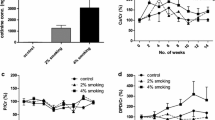
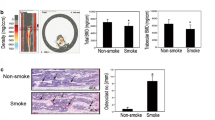
Cell migration and matrix remodeling are key events in tissue repair and restructuring. Osteoblasts are responsible for the production of new bone matrix during bone remodeling. The activity of these cells can be modulated by a number of factors. The current study evaluated the hypothesis that cigarette smoke extract can alter repair and remodeling responses of human osteoprogenitor cells and osteoblast-like cells and, therefore, could explain one mechanism by which cigarette smoking leads to osteoporosis. Human osteoprogenitor cells were isolated from normal human bone marrow and maintained in culture under either control conditions or conditions that induced differentiation into osteoblast-like cells. Both cell types migrated toward fibronectin and PDGF-BB as chemoattractants. Neither responded to TGF-β1. The osteoprogenitor cells were more active in their chemotactic response. The chemotactic response of both cell types was inhibited by cigarette smoke extract in a concentration-dependent manner. Both cell types, when cultured in three-dimensional native collagen gels maintained in floating culture, induced contraction of their surrounding matrices. Contraction was augmented by serum, PDGF-BB, and TGF-β1. Osteoprogenitor cells were less active in inducing contraction than were osteoblast-like cells. Contraction of both cell types was inhibited by cigarette smoke extract. Cigarette smoke extract also inhibited the production of fibronectin by both cell types maintained in three-dimensional culture. Addition of exogenous fibronectin partially restored the ability of the cells to contract three-dimensional collagen gels. The current study demonstrates that cigarette smoke can interfere with the ability of bone cells to participate in repair and remodeling events. Such an effect may be one mechanism leading to the development of osteoporosis.







Similar content being viewed by others
References
Doll R, Peto R, Wheatley K, Gray R, Sutherland I (1994) Mortality in relation to smoking: 40 years' observations on male British doctors [see comments]. Br Med J 309(6959):901–911
Rodan G, Raisz L, Bilezikian J (eds) (1996) Pathophysiology of osteoporosis, principles of bone biology. Academic Press, San Diego
Schmitz MA, Finnegan M, Natarajan R, Champine J (1999) Effect of smoking on tibial shaft fracture healing. Clin Orthop 365:184–200
Ward KD, Klesges RC (2001) A meta-analysis of the effects of cigarette smoking on bone mineral density. Calcif Tissue Int 68:259–270
Willey JC, Grafstrom RC, Moser CE Jr, Ozanne C, Sundqvist K, Harris CC (1987) Biochemical and morphological effects of cigarette smoke condensate and its fractions on normal human bronchial epithelial cells in vitro. Cancer Res 47:2045–2049
Wang H, Liu X, Umino R, Skold CM, Zhu Y, Kohyama T, Spurzem JR, Romberger DJ, Rennard SI (2001) Cigarette smoke inhibits human bronchial epithelial cell repair processes. Am J Respir Cell Mol Biol 25:772–779
Nakamura Y, Romberger DJ, Tate L, Ertl RF, Kawamoto M, Adachi Y, Mio T, Sisson JH, Spurzem JR, Rennard SI (1995) Cigarette smoke inhibits lung fibroblast proliferation and chemotaxis. Am J Respir Crit Care Med 151:1497–1503
Jones G, Riley M, Dwyer T (1999) Maternal smoking during pregnancy, growth, and bone mass in prepubertal children. J Bone Miner Res 14:146–151
Rapuri PB, Gallagher JC, Balhorn KE, Ryschon KL (2000) Smoking and bone metabolism in elderly women. Bone 27:429–436
Orwoll ES (1998) Osteoporosis in men. Endocrinol Metab Clin North Am 27:349–367
Ueng SW, Lee SS, Lin SS, Wang CR, Liu SJ, Tai CL, Shih CH (1999) Hyperbaric oxygen therapy mitigates the adverse effect of cigarette smoking on the bone healing of tibial lengthening: an experimental study on rabbits. J Trauma 47:752–759
Broulik PD, Jarab J (1993) The effect of chronic nicotine administration on bone mineral content in mice. Horm Metab Res 25:219–221
Iwaniec UT, Fung YK, Akhter MP, Haven MC, Nespor S, Haynatzki GR, Cullen DM (2001) Effects of nicotine on bone mass, turnover, and strength in adult female rats. Calcif Tissue Int 68:358–364
Kanno S, Anuradha CD, Hirano S (2001) Chemotactic responses of osteoblastic MC3T3-E1 cells toward zinc chloride. Biol Trace Elem Res 83:49–55
Lind M, Deleuran B, Yssel H, Fink-Eriksen E, Thestrup-Pedersen K (1995) Il-4 and IL-13, but not IL-10, are chemotactic factors for human osteoblasts. Cytokine 7:78–82
Kinoshita S, Finnegan M, Bucholz RW, Mizuno K (1999) Three-dimensional collagen gel culture promotes osteoblastic phenotype in bone marrow derived cells. Kobe J Med Sci 45:201-211
Talley-Ronsholdt DJ, Lajiness E, Nagodawithana K (1995) Transforming growth factor-beta inhibition of mineralization by neonatal rat osteoblasts in monolayer and collagen gel culture. In Vitro Cell Div Biol 31:274–282
Grinnell F (1994) Fibroblasts, myofibroblasts and wound contraction. J Cell Biol 124:401–404
Haynesworth SE, Goshima J, Goldberg VM, Caplan AI (1992) Characterization of cells with osteogenic potential from human marrow. Bone 13:81–88
Rickard DJ, Kassem M, Hefferan TE, Sarkar G, Spelsberg TC, Riggs BL (1996) Isolation and characterization of osteoblast precursor cells from human bone marrow. J. Bone Miner Res 11:312–324
Liu XD, Zhu YK, Umino T, Spurzem JR, Romberger DJ, Wang H, Reed E, Rennard SI (2001) Cigarette smoke inhibits osteogenic differentiation and proliferation of human osteoprogenitors in monolayer and three dimensional culture. J Lab Clin Med 137:208–219
Carp H, Janoff A (1978) Possible mechanisms of emphysema in smokers: in vitro suppression of serum elastase-inhibitory capacity by fresh cigarette smoke and its prevention by antioxidants. Am Rev Respir Dis 118:617–621
Ishii T, Matsuse T, Igarashi H, Masuda M, Teramoto S, Ouchi Y (2001) Tobacco smoke reduces viability in human lung fibroblasts: protective effect of glutathione S-transferase P1. Am J Physiol Lung Cell Mol Physiol 280:L1189–L1195
Hoshino Y, Mio T, Nagai S, Miki H, Ito I, Izumi T (2001) Cytotoxic effects of cigarette smoke extract on an alveolar type II cell-derived cell line. Am J Physiol Lung Cell Mol Physiol 281:L509–L516
Engvall E, Ruoslahti E (1977) Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer 20:1–5
Falk W, Goodwin RH, Leonard EJ (1980) A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods 33:239–247
Mio T, Liu X, Adachi Y, Striz I, Skold CM, Romberger DJ, Spurzem JR, Illig MG, Ertl R, Rennard SI (1998) Human bronchial epithelial cells modulate collagen gel contraction by fibroblasts. Am J Phys 274:L119-L126
Rennard SI, Berg R, Martin GR, Foidart JM, Robey PG (1980) Enzyme linked immunoassay (ELISA) for connective tissue components. Anal Biochem 104:205–214
Carnevali S, Nakamura Y, Mio T, Liu X, Takigawa K, Romberger DJ, Spurzem JR, Rennard SI (1998) Cigarette smoke extract inhibits fibroblast-mediated collagen gel contraction. Am J Phys 247:L591-L598
Buist AS, Vollmer WM (1994) Smoking and other risk factors. In: Murray JF, Nadel JA (eds) Textbook of respiratory medicine. Saunders, Philadelphia, pp 1259–1287
Hadley MN, Reddy SV (1997) Smoking and the human vertebral column: a review of the impact of cigarette use on vertebral bone metabolism and spinal fusion. Neurosurgery 41:116–124
Seeman E, Melton L, O'Fallon W, Riggs B (1983) Risk factors for spinal osteoporosis in men. Am J Med 75:977–983
Lofroth G (1989) Environmental tobacco smoke: overview of chemical composition and genotoxic components. Mutat Res 222:73–80
Sato E, Koyama S, Takamizawa A, Masubuchi T, Kubo K, Robbins RA, Nagai S, Izumi T (1999) Smoke extract stimulates lung fibroblasts to release neutrophil and monocyte chemotactic activities. Am J Physiol 277(6 Pt 1):L1149–L1157
Henningfield JE, Goldberg SR (1988) Progress in understanding the relationship between the pharmacological effects of nicotine and human tobacco dependence. Pharmacol Biochem Behav 30:217–220
Brandau S, Bohle A (2001) Bladder cancer: molecular and genetic basis of carcinogenesis. Eur Urol 39:491–497
Panagakos FS (1994) Transforming growth factor-alpha stimulates chemotaxis of osteoblasts and osteoblast-like cells in vitro. Biochem Mol Biol Int 33:643–650
Lind M, Deleuram B, Thestrup-Pedersen K, Soballe K, Eriksen EF, Bunger C (1995) Chemotaxis of human osteoblasts: effects of osteotropic growth factors. APMIS 103:140–146
Riikonen T, Koivisto L, Vihinen P, Heino J (1995) Transforming growth factor-β regulates collagen gel contraction by increasing α2β1 integrin expression in osteogenic cells. J Biol Chem 270:376–382
Asaga H, Kikuchi S, Yoshizato K (1991) Collagen gel contraction by fibroblasts requires cellular fibronectin but not plasma fibronectin. Exp Cell Res 193:167–174
Mochitate K, Pawelek P, Grinnell F (1991) Stress relaxation of contracted collagen gels: disruption of actin filament bundles, release of cell surface fibronectin, and down-regulation of DNA and protein synthesis. Exper Cell Res 193:198–207
Acknowledgements
The authors acknowledge the excellent secretarial support of Ms Lillian Richards and the editorial assistance of Ms Mary Tourek.
Author information
Authors and Affiliations
Corresponding author
Additional information
Funding source: Larson Endowment, University of Nebraska Medical Center, Omaha, Nebraska
Rights and permissions
About this article
Cite this article
Liu, X., Kohyama, T., Kobayashi, T. et al. Cigarette smoke extract inhibits chemotaxis and collagen gel contraction mediated by human bone marrow osteoprogenitor cells and osteoblast-like cells. Osteoporos Int 14, 235–242 (2003). https://doi.org/10.1007/s00198-002-1350-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-002-1350-7