Abstract
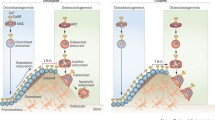
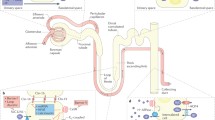
The extracellular calcium-sensing receptor (CaR) plays key roles in maintaining extracellular calcium homeostasis by enabling several of the cells and tissues involved in this process to sense small changes in Ca2+ o and to respond with changes in cellular function that will restore Ca2+ o to its normal level. The chief cells of the parathyroid gland and the thyroidal C-cells, for example, respond to decreases in Ca2+ o with increased secretion of the Ca2+ o-elevating hormone, parathyroid hormone (PTH), and decreased secretion of the Ca2+ o-lowering hormone, calcitonin, respectively. The cells of the renal distal tubule are likewise capable of sensing Ca2+ o and respond to decreases in Ca2+ o with increased tubular reabsorption of Ca2+ and vice versa, alterations in tubular function that will contribute to normalization of Ca2+ o. The skeleton also plays key roles in maintaining Ca2+ o homeostasis and both osteoblasts and osteoclasts can sense Ca2+ o, with elevations in Ca2+ o promoting bone formation and inhibiting bone resorption. It has been suggested that Sr2+ could act on bone via the CaR; however, the molecular mechanisms through which Ca2+ o and Sr2+ o exert these actions on bone cells remain controversial. Therefore, identifying their molecular target(s) would have significant implications for the treatment of bone loss. Ideally, therapies should simultaneously inhibit bone resorption while stimulating bone formation. Administration of strontium produces exactly those effects. Previous studies with dispersed bovine parathyroid cells as well as a preliminary study using CaR-transfected Chinese hamster ovary (CHO) cells indicate that Sr2+ o is an agonist of the CaR, albeit with slightly lower efficacies and potencies than Ca2+ o. Given that Sr2+ o is distributed preferentially in bone, therefore, an action of this divalent cation on the CaR in bone cells represents one possible mechanism by which strontium ranelate, a new antiosteoporotic drug, exerts it skeletal actions in vivo.
Similar content being viewed by others
References
Brown EM. Extracellular Ca2+ sensing, regulation of parathyroid cell function, and role of Ca2+ and other ions as extracellular (first) messengers. Physiol Rev 1991;71:371–411.
Berridge M, Lipp M, Bootman M. Calcium signalling. Curr Biol 1999;9:R157–9.
Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev 1994;74:595–636.
Brown EM. Physiology of Calcium homeostasis. In: Biliezikian JP, Raisz LG, Rodan G, editors. The parathyroids, 2nd ed. San Diego: Academic Press, 2001:167–81.
Bringhurst FR, Demay MB, Kronenberg HM. Hormones and disorders of mineral metabolism. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR, editors. Williams textbook of endocrinology, 9th ed. Philadelphia: WB Saunders, 1998:1155–209.
Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev 2001;81:239–97.
Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature 1993;366:575–80.
Marie PJ, Hott M, Modrowski D, De Pollak C, Guillemain J, Deloffre P, et al. An uncoupling agent containing strontium prevents bone loss by depressing bone resorption and maintaining bone formation in estrogen-deficient rats. J Bone Miner Res 1993;8:607–15.
Grynpas MD, Hamilton E, Cheung R, Tsouderos Y, Deloffre P, Hott M, et al. Strontium increases vertebral bone volume in rats at a low dose that does not induce detectable mineralization defect. Bone 1996;18:253–9.
Buehler J, Chappuis P, Saffar JL, Tsouderos Y, Vignery A. Strontium ranelate inhibits bone resorption while maintaining bone formation in alveolar bone in monkeys (Macaca fascicularis). Bone 2001;29:176–9.
Brown EM, Fuleihan Ge-H, Chen CJ, Kifor O. A comparison of the effects of divalent and trivalent cations on parathyroid hormone release, 3',5'-cyclic-adenosine monophosphate accumulation, and the levels of inositol phosphates in bovine parathyroid cells. Endocrinology 1990;127:1064–71.
Quarles LD. Cation-sensing receptors in bone: a novel paradigm for regulating bone remodeling? J Bone Miner Res 1997;12:1971–4.
Abou-Samra A, Juppner H, Force T, Freeman MW, Kong X, Schipani E, et al. Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol phosphates and increases intracellular free calcium. Proc Natl Acad Sci USA 1992;89:2732–6.
Bringhurst F, Juppner H, Guo J, Urena P, Potts JJ, Kronenberg H, et al. Cloned, stably expressed parathyroid hormone (PTH)/PTH-related peptide receptors activate multiple messenger signals and biological responses. Endocrinology 1993;132:2090–8.
Silver J, Sela SB, Naveh-Many T. Regulation of parathyroid cell proliferation. Curr Opin Nephrol Hypertens 1997;6:321–6.
Nemeth E, Scarpa A. Rapid mobilization of cellular Ca2+ in bovine parathyroid cells by external divalent cations. J Biol Chem 1987;202:5188–96.
Chen C, Barnett J, Congo D, Brown E. Divalent cations suppress 3',5'-adenosine monophosphate accumulation by stimulating a pertussis toxin-sensitive guanine nucleotide-binding protein in cultured bovine parathyroid cells. Endocrinology 1989;124:233–9.
Brown EM, Butters R, Katz C, Kifor O. Neomycin mimics the effects of high extracellular calcium concentrations on parathyroid function in dispersed bovine parathyroid cells. Endocrinology 1991;128:3047–54.
Racke F, Hammerland L, Dubyak G, Nemeth E. Functional expression of the parathyroid cell calcium receptor in Xenopus oocytes. FEBS Lett 1993;333:132–6.
Chen TH, Pratt SA, Shoback DM. Injection of bovine parathyroid poly(A)+ RNA into Xenopus oocytes confers sensitivity to high extracellular calcium. J Bone Miner Res 1994;9:293–300.
Riccardi D, Hall AE, Chattopadhyay N, Xu JZ, Brown EM, Hebert SC. Localization of the extracellular Ca2+/polyvalent cation-sensing protein in rat kidney. Am J Physiol 1998;274:F611–22.
Chattopadhyay N, Brown EM. Cellular "sensing" of extracellular calcium (Ca(2+) (o)): emerging roles in regulating diverse physiological functions. Cell Signal 2000;12:361–6.
Bai M, Trivedi S, Lane CR, Yang Y, Quinn SJ, Brown EM. Protein kinase C phosphorylation of threonine at position 888 in Ca2+ o-sensing receptor (CaR) inhibits coupling to Ca2+ store release. J Biol Chem 1998;273:21267–75.
Bai M. Structure and function of the extracellular calcium-sensing receptor [review]. Int J Mol Med 1999;4:115–25.
Ray K, Hauschild BC, Steinbach PJ, Goldsmith PK, Hauache O, Spiegel AM. Identification of the cysteine residues in the amino-terminal extracellular domain of the human Ca(2+) receptor critical for dimerization. Implications for function of monomeric Ca(2+) receptor. J Biol Chem 1999;274:27642–50.
Sharff AJ, Rodseth LE, Spurlino JC, Quiocho FA. Crystallographic evidence for a large ligand-induced hinge-twist motion between the two domains of the maltodextrin binding protein involved in active transport and chemotaxis. Biochemistry 1992;31:10657–63.
Kifor O, Diaz R, Butters R, Brown EM. The Ca2+-sensing receptor (CaR) activates phospholipases C, A2, and D in bovine parathyroid and CaR-transfected, human embryonic kidney (HEK293) cells. J Bone Miner Res 1997;12:715–25.
McNeil SE, Hobson SA, Nipper V, Rodland KD. Functional calcium-sensing receptors in rat fibroblasts are required for activation of SRC kinase and mitogen-activated protein kinase in response to extracellular calcium. J Biol Chem 1998;273:1114–20.
Arthur JM, Lawrence MS, Payne CR, Rane MJ, McLeish KR. The calcium-sensing receptor stimulates JNK in MDCK cells. Biochem Biophys Res Commun 2000;275:538–41.
Pollak MR, Brown EM, Chou YH, Hebert SC, Marx SJ, Steinmann B, et al. Mutations in the human Ca(2+)-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell 1993;75:1297–303.
Pollak MR, Chou YH, Marx SJ, Steinmann B, Cole DE, Brandi ML, Papapoulos SE, Menko FH, Hendy GN, Brown EM, et al. Familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Effects of mutant gene dosage on phenotype. J Clin Invest 1994;93:1108–12.
Ho C, Conner DA, Pollak MR, Ladd DJ, Kifor O, Warren HB, Brown EM, Seidman JG, Seidman CE. A mouse model of human familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Nat Genet 1995;11:389–94.
Corbetta S, Lania A, Filopanti M, Vincentini L, Ballare E, Spada A. Mitogen-activated protein kinase cascade in human normal and tumoral parathyroid cells. J Clin Endocrinol Metab 2002;87:2201–5.
Wada M, Nagano N, Furuya Y, Chin J, Nemeth EF, Fox J. Calcimimetic NPS R-568 prevents parathyroid hyperplasia in rats with severe secondary hyperparathyroidism. Kidney Int 2000;57:50–8.
Garrett J, Steffey M, Nemeth E. The calcium receptor agonist R-568 suppresses PTH mRNA levels in cultured bovine parathyroid cells [abstract]. J Bone Miner Res 1995;10 (Suppl. 1):S387.
Eskert R, Scherubl H, Petzelt C, Friedhelm R, Ziegler R. Rhythmic oscillations of cytosolic calcium in rat C-cells. Mol Cell Endocrinol 1989;64:267–270.
Muff R, Nemeth EF, Haller-Brem S, Fischer JA. Regulation of hormone secretion and cytosolic Ca2+ by extracellular Ca2+ in parathyroid cells and C-cells: role of voltage-sensitive Ca2+ channels. Arch Biochem Biophys 1988;265:128–35.
Garrett JE, Tamir H, Kifor O, Simin RT, Rogers KV, Mithal A, et al. Calcitonin-secreting cells of the thyroid express an extracellular calcium receptor gene. Endocrinology 1995;136:5202–11.
Freichel M, Zink-Lorenz A, Holloschi A, Hafner M, Flockerzi V, Raue F. Expression of a calcium-sensing receptor in a human medullary thyroid carcinoma cell line and its contribution to calcitonin secretion. Endocrinology 1996;137:3842–8.
Hebert SC, Brown EM, Harris HW. Role of the Ca(2+)-sensing receptor in divalent mineral ion homeostasis. J Exp Biol 1997;200:295–302.
Wang WH, Lu M, Hebert SC. Cytochrome P-450 metabolites mediate extracellular Ca(2+)-induced inhibition of apical K+ channels in the TAL. Am J Physiol 1996;271:C103–11.
Bapty BW, Dai LJ, Ritchie G, Jirik F, Canaff L, Hendy GN, et al. Extracellular Mg2(+)- and Ca2(+)-sensing in mouse distal convoluted tubule cells. Kidney Int 1998;53:583–92.
Peng J-B, Chen XZ, Berger UV, Vassilev PM, Tsukaguchi H, Brown EM, et al. Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J Biol Chem 1999;274:22739–46.
Hoenderop JGJ, Van de Graaf AWCM, Hartog A, van de Graaf SFJ, van Os CH, Willems PHGM, et al. Molecular identification of the apical Ca2+ channel in 1,25-dihydroxyvitamin D3-responsive epithelia. J Biol Chem 1999;274:8375–8.
Silver IA, Murrils RJ, Etherington DJ. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res 1988;175:266–76.
Yamaguchi T, Chattopadhyay N, Brown EM. G protein-coupled extracellular Ca2+ (Ca2+ o)-sensing receptor (CaR): roles in cell signaling and control of diverse cellular functions. Adv Pharmacol 2000;47:209–53.
Yamaguchi T, Chattopadhyay N, Kifor O, Butters RR Jr, Sugimoto T, Brown EM. Mouse osteoblastic cell line (MC3T3-E1) expresses extracellular calcium (Ca2+ o)-sensing receptor and its agonists stimulate chemotaxis and proliferation of MC3T3-E1 cells. J Bone Miner Res 1998;13:1530–8.
Lajeunesse D, Kiebzak GM, Frondoza C, Sacktor B. Regulation of osteocalcin secretion by human primary bone cells and by the human osteosarcoma cell line MG-63. Bone Miner 1991;14:237–50.
Raisz LG, Niemann I. Effect of phosphate, calcium, and magnesium on bone resorption and bone formation in tissue culture. Endocrinology 1969;85:446–52.
House MG, Kohlmeier L, Chattopadhyay N, Kifor O, Yamaguchi T, Leboff MS, et al. Expression of an extracellular calcium-sensing receptor in human and mouse bone marrow cells. J Bone Miner Res 1997;12:1959–70.
Chang W, Tu C, Chen T-H, Komuves L, Oda Y, Pratt S, et al. Expression and signal transduction of calcium-sensing receptors in cartilage and bone. Endocrinology 1999;140:5883–93.
Quarles DL, Hartle II JE, Siddhanti SR, Guo R, Hinson TK. A distinct cation-sensing mechanism in MC3T3-E1 osteoblasts functionally related to the calcium receptor. J Bone Miner Res 1997;12:393–402.
Pi M, Hinson TK, Quarles L. Failure to detect the extracellular calcium-sensing receptor (CasR) in human osteoblast cell lines. J Bone Miner Res 1999;14:1310–9.
Pi M, Garner SC, Flannery P, Spurney RF, Quarles LD. Sensing of extracellular cations in CasR-deficient osteoblasts. Evidence for a novel cation-sensing mechanism. J Biol Chem 2000;275:3256–63.
Olszak IT, Poznansky MC, Evans RH, Olson D, Kos C, Pollak MR, et al. Extracellular calcium elicits a chemokinetic response from monocytes in vitro and in vivo. J Clin Invest 2000;105:1299–305.
Yamaguchi T, Olozak I, Chattopadhyay N, Butters RR, Kifor O, Scadden DT, et al. Expression of extracellular calcium (Ca2+ o)-sensing receptor in human peripheral blood monocytes. Biochem Biophys Res Commun 1998;246:501–6.
Kanatani M, Sugimoto T, Kanzawa M, Yano S, Chihara K. High extracellular calcium inhibits osteoclast-like cell formation by directly acting on the calcium-sensing receptor existing in osteoclast precursor cells. Biochem Biophys Res Commun 1999;261:144–8.
Yamaguchi T, Chattopadhyay N, Brown EM. G protein-coupled extracellular Ca2+ (Ca2+ o)-sensing receptor (CaR): roles in cell signaling and control of diverse cellular functions. Adv Pharmacol 1999;47:209–53.
Kameda T, Mano H, Yamada Y, Takai H, Amizuka N, Kobori M, et al. Calcium-sensing receptor in mature osteoclasts, which are bone resorbing cells. Biochem Biophys Res Commun 1998;245:419–22.
Zaidi M, Adebanjo OA, Moonga BS, Sun L, Huang CL. Emerging insights into the role of calcium ions in osteoclast regulation. J Bone Miner Res 1999;14:669–74.
Canalis E, Hott M, Deloffre P, Tsouderos Y, Marie PJ. The divalent strontium salt S 12911 enhances bone cell replication and bone formation in vitro. Bone 1996;18:517–23.
Boivin G, Deloffre P, Perrat B, Panczer G, Boudeulle M, Mauras Y, et al. Strontium distribution and interactions with bone mineral in monkey iliac bone after strontium salt (S 12911) administration. J Bone Miner Res 1996;11:1302–11.
Dahl SG, Allain P, Marie PJ, Mauras Y, Boivin G, Ammann P, et al. Incorporation and distribution of strontium in bone. Bone 2001;28:446–453.
Meunier PJ, Slosman DO, Delmas PD, Sebert JL, Brandi ML, Albanese C, et al. Strontium ranelate: dose-dependent effects in established postmenopausal vertebral osteoporosis. The STRATOS 2-year randomized placebo controlled trial. J Clin Endocrinol Metab 2002;87:2060–6.
Coulombe J, Faure H, Robin B, Tsouderos Y, Ruat M. Stimulatory effects of strontium ranelate (S12911) on the rat and mouse cation-sensing receptor. J Bone Miner Res 2001;16(Suppl 1):SU500.
Coulombe J, Faure H, Robin B, Tsouderos Y, Ruat M. Activation of the rat and mouse cation-sensing receptor by strontium ranelate and its modulation by extracellular calcium. Osteoporos Int 2002;13(Suppl 1):P70MO.
Acknowledgement
The author receives research support from the Institut de Recherches Internationales Servier.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brown, E.M. Is the calcium receptor a molecular target for the actions of strontium on bone?. Osteoporos Int 14 (Suppl 3), 25–34 (2003). https://doi.org/10.1007/s00198-002-1343-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-002-1343-6