Abstract
Introduction and hypothesis
In 2008 and 2011, the US Food and Drug Administration (FDA) released notifications regarding vaginal mesh. In describing prolapse surgery trends over time, we predicted vaginal mesh use would decrease and native tissue repairs would increase.
Methods
Operative reports were reviewed for all prolapse repairs performed from 2008 to 2011 at our large regional hospital system. The number of each type of prolapse repair was determined per quarter year and expressed as a percentage of all repairs. Surgical trends were examined focusing on changes with respect to the release of two FDA notifications. We used linear regression to analyze surgical trends and chi-square for demographic comparisons.
Results
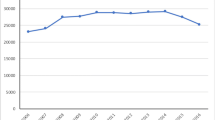
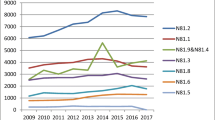
One thousand two hundred and eleven women underwent 1,385 prolapse procedures. Mean age was 64 ± 12, and 70 % had stage III prolapse. Vaginal mesh procedures declined over time (p = 0.001), comprising 27 % of repairs in early 2008, 15 % at the first FDA notification, 5 % by the second FDA notification, and 2 % at the end of 2011. The percentage of native tissue anterior/posterior repairs (p < 0.001) and apical suspensions (p = 0.007) increased, whereas colpocleisis remained constant (p = 0.475). Despite an overall decrease in open sacral colpopexies (p < 0.001), an initial increase was seen around the first FDA notification. We adopted laparoscopic/robotic techniques around this time, and the percentage of minimally invasive sacral colpopexies steadily increased thereafter (p < 0.001). All sacral colpopexies combined as a group declined over time (p = 0.011).
Conclusions
Surgical treatment of prolapse continues to evolve. Over a 4-year period encompassing two FDA notifications regarding vaginal mesh and the introduction of laparoscopic/robotic techniques, we performed fewer vaginal mesh procedures and more native tissue repairs and minimally invasive sacral colpopexies.



Similar content being viewed by others
References
Lane FE (1962) Repair of posthysterectomy vaginal-vault prolapse. Obstet Gynecol 20:72–77
Maher C, Baessler K, Glazener CM, Adams EJ, Hagen S (2004) Surgical management of pelvic organ prolapse. Cochrane Database Syst Rev 4, CD004014
Food and Drug Administration (2011) Urogynecologic surgical mesh: update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse. Silver Springs (MD): FDA. Available at: http://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM262760.pdf. Retrieved July 30, 2013
Jonsson Funk M, Edenfield AL, Patet V, Visco AG, Weidner AC, Wu JM (2013) Trends in use of surgical mesh for pelvic organ prolapse. Am J Obstet Gynecol 208:79.e1–7
Abed H, Rahn DD, Lowenstein L, Balk EM, Clemons JL, Rogers RG (2011) Incidence and management of graft erosion, wound granulation, and dyspareunia following vaginal prolapse repair with graft materials: a systematic review. Int Urogynecol J 22:789–798
Sokol AI, Iglesia CB, Kudish BI, Gutman RE, Shveiky D, Bercik R, Sokol ER (2012) One-year objective and functional outcomes of a randomized clinical trial of vaginal mesh for prolapse. Am J Obstet Gynecol 206:86.e1–86.e9
Food and Drug Administration (2008) FDA public health notification: serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. Silver Spring (MD): FDA. Available at: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm. Retrieved Jan 7, 2012
Food and Drug Administration (2011) FDA safety communication: UPDATE on serious complications associated with transvaginal placement of surgical mesh for pelvic organ prolapse. Silver Spring (MD): FDA. Available at: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm262435.htm. Retrieved Jan 7, 2012
Committee on Gynecologic Practice (2012) Vaginal placement of synthetic mesh for pelvic organ prolapse. Female Pelvic Med Reconstr Surg 18:5–9
Davila GW, Baessler K, Cosson M, Cardozo L (2012) Selection of patients in whom vaginal graft use may be appropriate. Consensus of the second IUGA Grafts Roundtable: optimizing safety and appropriateness of graft use in transvaginal pelvic reconstructive surgery. Int Urogynecol J 23:s7–s14
Miller D, Milani AL, Sutherland SE, Navin B, Rogers RG (2012) Informed surgical consent for a mesh/graft-augmented vaginal repair of pelvic organ prolapse. Consensus of the second IUGA Grafts Roundtable: optimizing safety and appropriateness of graft use in transvaginal pelvic reconstructive surgery. Int Urogynecol J 23:s33–s42
Paraiso MF, Walters MD, Rackley RR, Melek S, Hugney C (2005) Laparoscopic and abdominal sacral colpopexies: a comparative cohort study. Am J Obstet Gynecol 192:1752–1758
Fitzgerald MP, Richter HE, Bradley CS, Ye W, Visco AC, Cundiff GW, Zyczynski HM, Fine P, Weber AM (2008) Pelvic support, pelvic symptoms, and patient satisfaction after colpocleisis. Int Urogynecol J 19:1603–1609
Wyatt J (1912) Le Fort’s operation for prolapse, with an account of eight cases. J Obstet Gynaecol Br Emp 22:266–269
Nygaard I, Brubaker L, Zyczynski HM, Cundiff G, Richter H, Gantz M, Fine P, Menefee S, Ridgeway B, Visco A, Warren LK, Zhang M, Meikle S (2013) Long-term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse. JAMA 309:2016–2024
Freeman RM, Pantazis K, Thomson A, Frappell J, Bombieri L, Moran P, Slack M, Scot P, Waterfield M (2013) A randomized controlled trial of abdominal versus laparoscopic sacrocolpopexy for the treatment of post-hystereectomy vaginal vault prolapse: LAS study. Int Urogynecol J 24:377–384
Paraiso MF, Jelovsek JE, Frick A, Chen CC, Barber MD (2011) Laproscopic compared with robotic sacrocolpopexy for vaginal prolapse: a randomized controlled trial. Obstet Gynecol 118:1005–1013
Rogo-Gupta L, Rodriguez LV, Litwin MS, Herzog TJ, Neugut AI, Lu YS, Raz S, Hershman DL, Wright JD (2012) Trends in surgical mesh use for pelvic organ prolapse from 2000 to 2010. Obstet Gynecol 120:1105–1115
Araco F, Gravante G, Sorge R, De Vita D, Piccione E (2008) Risk evaluation of smoking and age on the occurrence of postoperative erosions after transvaginal mesh repair for pelvic organ prolapses. Int Urogynecol J Pelvic Floor Dysfunct 19:473–479
Araco F, Gravante G, Sorge R, Overton J, De Vita D, Primicerio M, Dati S, Araco P, Piccione E (2009) The influence of BMI, smoking, and age on vaginal erosions after synthetic mesh repair of pelvic organ prolapses. A multicenter study. Acta Obstet Gynecol Scand 88:772–780
Elmér C, Falconer C, Hallin A, Larsson G, Ek M, Altman D, Nordic Transvaginal Mesh Group (2012) Risk factors for mesh complications after trocar guided transvaginal mesh kit repair of anterior vaginal wall prolapse. Neurourol Urodyn 31:1165–1169
Lowman JK, Woodman PJ, Nosti PA, Bump RC, Terry CL, Hale DS (2008) Tobacco use is a risk factor for mesh erosion after abdominal sacral colpoperineopexy. Am J Obstet Gynecol 198:561.e1–561.e4
Diwadkar GB, Barber MD, Feiner B, Maher C, Jelovsek JE (2009) Complication and reoperation rates after apical vaginal prolapse surgical repair: a systematic review. Obstet Gynecol 113:367–373
Ehsani N, Ghafar MA, Antosh DD, Tan-Kim J, Warner WB, Mamik MM, Brown HW, Chung CP, Segal S, Abed H, Murphy M, Stolzfus JC, Molden SM (2012) Risk factors for mesh extrusion after prolapse surgery: a case–control study. Female Pelvic Med Reconstr Surg 18:357–361
Acknowledgments
This project was supported by the National Institutes of Health through grant numbers UL1 RR024153 and UL1 TR000005. We thank Dr. Neena Agarwala for her assistance with data collection.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presentation Information
These findings were presented at the 33rd Annual Meeting of the American Urogynecologic Society, Chicago, IL, USA, October 2012.
Rights and permissions
About this article
Cite this article
Skoczylas, L.C., Turner, L.C., Wang, L. et al. Changes in prolapse surgery trends relative to FDA notifications regarding vaginal mesh. Int Urogynecol J 25, 471–477 (2014). https://doi.org/10.1007/s00192-013-2231-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-013-2231-7