Abstract
Introduction and hypothesis
We assessed fesoterodine efficacy and tolerability in women with overactive bladder (OAB).
Methods
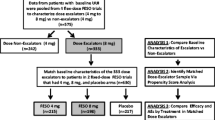
This post hoc analysis of pooled data from two clinical trials included 1,548 women with OAB randomized to placebo, fesoterodine 4 or 8 mg, or tolterodine extended release (ER) 4 mg (in 1 trial) for 12 weeks. Subjects completed 3-day bladder diaries at baseline and weeks 2 and 12 and rated Treatment Response at weeks 2 and 12.
Results
By weeks 2 and 12, all active-treatment groups showed significant improvements in all five bladder diary variables assessed and greater Treatment Response rates vs placebo. Fesoterodine 8 mg was significantly more efficacious than fesoterodine 4 mg and tolterodine ER in improving urgency urinary incontinence episodes and continent days per week. The most common adverse events were dry mouth and constipation, which were predominately mild or moderate.
Conclusions
Fesoterodine is efficacious and well tolerated in women with OAB.

Similar content being viewed by others
Abbreviations
- 5-HMT:
-
5-Hydroxymethyl tolterodine
- AE:
-
Adverse event
- ANCOVA:
-
Analysis of covariance
- CYP:
-
Cytochrome P450
- EOT:
-
End of treatment
- ER:
-
Extended release
- FESO:
-
Fesoterodine
- HRQL:
-
Health-related quality of life
- LOCF:
-
Last observation carried forward
- LSM:
-
Least squares mean
- OAB:
-
Overactive bladder
- MVV:
-
Mean voided volume
- PBO:
-
Placebo
- SD:
-
Standard deviation
- SEM:
-
Standard error of the mean
- TOL ER:
-
Tolterodine ER
- UUI:
-
Urgency urinary incontinence
References
Irwin DE, Milsom I, Hunskaar S, Reilly K, Kopp Z, Herschorn S et al (2006) Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol 50:1306–15
Hu TW, Wagner TH, Bentkover JD, Leblanc K, Zhou SZ, Hunt T (2004) Costs of urinary incontinence and overactive bladder in the United States: a comparative study. Urology 63:461–5
Stach-Lempinen B, Sintonen H, Kujansuu E (2004) The relationship between clinical parameters and health-related quality of life as measured by the 15D in incontinent women before and after treatment. Acta Obstet Gynecol Scand 83:983–8
van der Vaart CH, de Leeuw JR, Roovers JP, Heintz AP (2002) The effect of urinary incontinence and overactive bladder symptoms on quality of life in young women. BJU Int 90:544–9
Coyne KS, Sexton CC, Irwin DE, Kopp ZS, Kelleher CJ, Milsom I (2008) The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: results from the EPIC study. BJU Int 101:1388–95
Coyne KS, Margolis MK, Jumadilova Z, Bavendam T, Mueller E, Rogers R (2007) Overactive bladder and women’s sexual health: what is the impact? J Sex Med 4:656–66
Chapple CR, Khullar V, Gabriel Z, Muston D, Bitoun CE, Weinstein D (2008) The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta-analysis. Eur Urol 54:543–62
Rogers R, Bachmann G, Jumadilova Z, Sun F, Morrow JD, Guan Z et al (2008) Efficacy of tolterodine on overactive bladder symptoms and sexual and emotional quality of life in sexually active women. Int Urogynecol J Pelvic Floor Dysfunct 19:1551–7
Elinoff V, Bavendam T, Glasser DB, Carlsson M, Eyland N, Roberts R (2006) Symptom-specific efficacy of tolterodine extended release in patients with overactive bladder: the IMPACT trial. Int J Clin Pract 60:745–51
Khullar V, Hill S, Laval K-U, Schiotz HA, Jonas U, Versi E (2004) Treatment of urge-predominant mixed urinary incontinence with tolterodine extended release: a randomized, placebo-controlled trial. Urology 64:269–75
Michel MC (2008) Fesoterodine: a novel muscarinic receptor antagonist for the treatment of overactive bladder syndrome. Expert Opin Pharmacother 9:1787–96
Malhotra B, Guan Z, Wood N, Gandelman K (2008) Pharmacokinetic profile of fesoterodine. Int J Clin Pharmacol Ther 46:556–63
Sachse R, Cawello W, Horstmann R (2004) Safety, tolerability and pharmacokinetics of fesoterodine after co-treatment with the potent cytochrome P450 3A4 inhibitor ketoconazole. Paper presented at International Continence Society Annual Meeting, Paris, France, August 25–27, 2004
Chapple C, van Kerrebroeck P, Tubaro A, Haag-Molkenteller C, Forst H-T, Massow U et al (2007) Clinical efficacy, safety, and tolerability of once-daily fesoterodine in subjects with overactive bladder. Eur Urol 52:1204–12
Nitti V, Dmochowski R, Sand P, Forst H-T, Haag-Molkenteller C, Massow U et al (2007) Efficacy, safety, and tolerability of fesoterodine in subjects with overactive bladder. J Urol 178:2488–94
Khullar V, Rovner ES, Dmochowski R, Nitti V, Wang J, Guan Z (2008) Fesoterodine dose response in subjects with overactive bladder syndrome. Urology 71:839–43
Kelleher CJ, Tubaro A, Wang JT, Kopp Z (2008) Impact of fesoterodine on quality of life: pooled data from two randomized trials. BJU Int 102:56–61
Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U et al (2003) The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 61:37–49
Colman S, Chapple C, Nitti V, Haag-Molkenteller C, Hastedt C, Massow U (2008) Validation of treatment benefit scale for assessing subjective outcomes in treatment of overactive bladder. Urology 72:803–07
Cohen J (1988) Statistical power analysis for the behavioral sciences. Lawrence Erlbaum, Hillsdale
Herschorn S, Heesakkers J, Castro-Diaz D, Wang JT, Brodsky M, Guan Z (2008) Effects of tolterodine extended release on patient perception of bladder condition and overactive bladder symptoms. Curr Med Res Opin 24:3513–21
Sussman DO, Kraus SR, Carlsson M, Guan Z (2007) Onset of efficacy of tolterodine extended release in patients with overactive bladder. Curr Med Res Opin 23:777–81
Irwin DE, Abrams P, Milsom I, Kopp Z, Reilly K (2008) Understanding the elements of overactive bladder: questions raised by the EPIC study. BJU Int 101:1381–7
Homma Y, Koyama N (2006) Minimal clinically important change in urinary incontinence detected by a quality of life assessment tool in overactive bladder syndrome with urge incontinence. Neurourol Urodyn 25:228–35
Cardozo L, Lisec M, Millard R, van Vierssen Trip O, Kuzmin I, Drogendijk TE et al (2004) Randomized, double-blind placebo controlled trial of the once daily antimuscarinic agent solifenacin succinate in patients with overactive bladder. J Urol 172:1919–24
Corcos J, Casey R, Patrick A, Andreou C, Miceli PC, Reiz JL et al (2006) A double-blind randomized dose-response study comparing daily doses of 5, 10 and 15 mg controlled-release oxybutynin: balancing efficacy with severity of dry mouth. BJU Int 97:520–7
Hill S, Khullar V, Wyndaele JJ, Lheritier K (2006) Dose response with darifenacin, a novel once-daily M3 selective receptor antagonist for the treatment of overactive bladder: results of a fixed dose study. Int Urogynecol J Pelvic Floor Dysfunct 17:239–47
Chapple CR, Van Kerrebroeck PE, Junemann KP, Wang JT, Brodsky M (2008) Comparison of fesoterodine and tolterodine in patients with overactive bladder. BJU Int 102:1128–32
Coyne KS, Elinoff V, Gordon DA, Deng DY, Brodsky M, Glasser DB et al (2008) Relationships between improvements in symptoms and patient assessments of bladder condition, symptom bother and health-related quality of life in patients with overactive bladder treated with tolterodine. Int J Clin Pract 62:925–31
Abrams P, Artibani W, Gajewski JB, Hussain I (2006) Assessment of treatment outcomes in patients with overactive bladder: importance of objective and subjective measures. Urology 68(suppl 2A):17–28
Acknowledgments
Editorial assistance was provided by Nancy Sheridan from Complete Healthcare Communications, Inc., and was funded by Pfizer Inc.
Funding
Funding for this study was provided by Schwarz BioSciences GmbH and Pfizer Inc.
Conflicts of interest
Peter Sand is an advisor for Astellas, Allergan, American Medical Systems, Boston Scientific, Coloplast, GlaxoSmithKline, Ortho McNeil, Pfizer Inc, and Watson Pharma; an investigator for Allergan, Boston Scientific, Ortho McNeil, Pfizer Inc, and Watson Pharma and a speaker for Allergan, Astellas, GlaxoSmithKline, Ortho McNeil, and Watson Pharma. Jon Morrow and Tamara Bavendam are employees of Pfizer Inc. Dana Creanga is a consultant for Pfizer Inc. Victor Nitti is an investigator for Schwarz Pharma, a consultant and lecturer for Pfizer Inc and Novartis, a consultant and investigator for Allergan, a consultant for Astellas, an advisor for Watson Pharma, Serenity Pharmaceuticals, and Coloplast Corp, and a lecturer for American Medical Systems.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sand, P.K., Morrow, J.D., Bavendam, T. et al. Efficacy and tolerability of fesoterodine in women with overactive bladder. Int Urogynecol J 20, 827–835 (2009). https://doi.org/10.1007/s00192-009-0857-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-009-0857-2