Abstract
Purpose
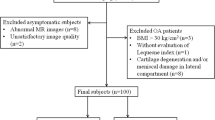
In order for T2 mapping to become more clinically applicable, reproducible subregions and standardized T2 parameters must be defined. This study sought to: (1) define clinically relevant subregions of knee cartilage using bone landmarks identifiable on both MR images and during arthroscopy and (2) determine healthy T2 values and T2 texture parameters within these subregions.
Methods
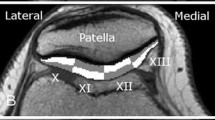
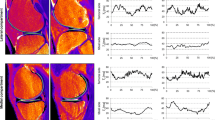
Twenty-five asymptomatic volunteers (age 18–35) were evaluated with a sagittal T2 mapping sequence. Manual segmentation was performed by three raters, and cartilage was divided into twenty-one subregions modified from the International Cartilage Repair Society Articular Cartilage Mapping System. Mean T2 values and texture parameters (entropy, variance, contrast, homogeneity) were recorded for each subregion, and inter-rater and intra-rater reliability was assessed.
Results
The central regions of the condyles had significantly higher T2 values than the posterior regions (P < 0.05) and higher variance than the posterior region on the medial side (P < 0.001). The central trochlea had significantly greater T2 values than the anterior and posterior condyles. The central lateral plateau had lower T2 values, lower variance, higher homogeneity, and lower contrast than nearly all subregions in the tibia. The central patellar regions had higher entropy than the superior and inferior regions (each P ≤ 0.001). Repeatability was good to excellent for all subregions.
Conclusion
Significant differences in mean T2 values and texture parameters were found between subregions in this carefully selected asymptomatic population, which suggest that there is normal variation of T2 values within the knee joint. The clinically relevant subregions were found to be robust as demonstrated by the overall high repeatability.




Similar content being viewed by others
References
(CDC) CfDCaP (2009) Prevalence and most common causes of disability among adults-United States, 2005. Morb Mortal Wkly Rep 58(16):421–426
Apprich S, Mamisch TC, Welsch GH, Stelzeneder D, Albers C, Totzke U, Trattnig S (2010) Quantitative T2 mapping of the patella at 3.0T is sensitive to early cartilage degeneration, but also to loading of the knee. Eur J Radiol 81(4):438–443
Blumenkrantz G, Stahl R, Carballido-Gamio J, Zhao S, Lu Y, Munoz T, Hellio Le Graverand-Gastineau MP, Jain SK, Link TM, Majumdar S (2008) The feasibility of characterizing the spatial distribution of cartilage T2 using texture analysis. Osteoarthritis Cartilage 16(5):584–590
Brittberg M, Winalski CS (2003) Evaluation of cartilage injuries and repair. J Bone Joint Surg Am 85-A(Suppl 2):58–69
Burstein D, Gray M, Mosher T, Dardzinski B (2009) Measures of molecular composition and structure in osteoarthritis. Radiol Clin North Am 47(4):675–686
Carballido-Gamio J, Link TM, Majumdar S (2008) New techniques for cartilage magnetic resonance imaging relaxation time analysis: texture analysis of flattened cartilage and localized intra- and inter-subject comparisons. Magn Reson Med 59(6):1472–1477
Carballido-Gamio J, Stahl R, Blumenkrantz G, Romero A, Majumdar S, Link TM (2009) Spatial analysis of magnetic resonance T1rho and T2 relaxation times improves classification between subjects with and without osteoarthritis. Med Phys 36(9):4059–4067
Chang G, Wiggins GC, Xia D, Lattanzi R, Madelin G, Raya JG, Finnerty M, Fujita H, Recht MP, Regatte RR (2011) Comparison of a 28-channel receive array coil and quadrature volume coil for morphologic imaging and T2 mapping of knee cartilage at 7T. J Magn Reson Imaging 35(2):441–448
Crema MD, Roemer FW, Marra MD, Burstein D, Gold GE, Eckstein F, Baum T, Mosher TJ, Carrino JA, Guermazi A (2011) Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics 31(1):37–61
Fleiss JL (1986) The design and analysis of clinical experiments. Wiley series in probability and mathematical statistics Applied probability and statistics. Wiley, New York, pp 1–35
Fragonas E, Mlynarik V, Jellus V, Micali F, Piras A, Toffanin R, Rizzo R, Vittur F (1998) Correlation between biochemical composition and magnetic resonance appearance of articular cartilage. Osteoarthritis Cartilage 6(1):24–32
Friedrich K, Shepard T, Chang G, Wang L, Babb J, Schweitzer M, Regatte R (2010) Does joint alignment affect the T2 values of cartilage in patients with knee osteoarthritis? Eur J Radiol 20(6):1532–1538
Friedrich KM, Shepard T, de Oliveira VS, Wang L, Babb JS, Schweitzer M, Regatte R (2009) T2 measurements of cartilage in osteoarthritis patients with meniscal tears. AJR Am J Roentgenol 193(5):W411–W415
Gluer CC, Blake G, Lu Y, Blunt BA, Jergas M, Genant HK (1995) Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos Int 5(4):262–270
Hannila I, Nieminen MT, Rauvala E, Tervonen O, Ojala R (2007) Patellar cartilage lesions: comparison of magnetic resonance imaging and T2 relaxation-time mapping. Acta Radiol 48(4):444–448
Haralick RM, Shanmugam K, Dinstein I (1973) Textural features for image classification. IEEE Trans Biomed Eng SMC-3(6):610–621
Haralick RM, Shanmugam K, Dinstein I (1973) Textural features for image classification. IEEE Trans Biomed Eng SMC-3:610–618
Hovis KK, Stehling C, Souza RB, Haughom BD, Baum T, Nevitt M, McCulloch C, Lynch JA, Link TM (2011) Physical activity is associated with magnetic resonance imaging-based knee cartilage T2 measurements in asymptomatic subjects with and those without osteoarthritis risk factors. Arthritis Rheum 63(8):2248–2256
Islam K, Duke K, Mustafy T, Adeeb SM, Ronsky JL, El-Rich M (2013) A geometric approach to study the contact mechanisms in the patellofemoral joint of normal versus patellofemoral pain syndrome subjects. Comput Methods Biomech Biomed Eng. doi:10.1080/10255842.2013.803082
Joseph G, Baum T, Carballido-Gamio J, Nardo L, Virayavanich W, Alizai H, Lynch J, McCulloch C, Majumdar S, Link T (2011) Texture analysis of cartilage T2 maps: individuals with risk factors for OA have higher and more heterogeneous knee cartilage MR T2 compared to normal controls-data from the osteoarthritis initiative. Arthritis Res Ther 13(5):R153
Li X, Benjamin Ma C, Link TM, Castillo DD, Blumenkrantz G, Lozano J, Carballido-Gamio J, Ries M, Majumdar S (2007) In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage 15(7):789–797
Li X, Pai A, Blumenkrantz G, Carballido-Gamio J, Link T, Ma B, Ries M, Majumdar S (2009) Spatial distribution and relationship of T1ρ and T2 relaxation times in knee cartilage with osteoarthritis. Magn Reson Med 61(6):1310–1318
Lusse S, Claassen H, Gehrke T, Hassenpflug J, Schunke M, Heller M, Gluer CC (2000) Evaluation of water content by spatially resolved transverse relaxation times of human articular cartilage. Magn Reson Imaging 18(4):423–430
Maher SA, Rodeo SA, Potter HG, Bonassar LJ, Wright TM, Warren RF (2011) A pre-clinical test platform for the functional evaluation of scaffolds for musculoskeletal defects: the meniscus. HSS J 7(2):157–163
Mamisch TC, Trattnig S, Quirbach S, Marlovits S, White LM, Welsch GH (2010) Quantitative T2 mapping of knee cartilage: differentiation of healthy control cartilage and cartilage repair tissue in the knee with unloading—initial results. Radiology 254(3):818–826
Nishioka H, Hirose J, Nakamura E, Okamoto N, Karasugi T, Taniwaki T, Okada T, Yamashita Y, Mizuta H (2013) Detecting ICRS grade 1 cartilage lesions in anterior cruciate ligament injury using T1rho and T2 mapping. Eur J Radiol 82(9):1499–1505
Pelletier JP, Raynauld JP, Berthiaume MJ, Abram F, Choquette D, Haraoui B, Beary JF, Cline GA, Meyer JM, Martel-Pelletier J (2007) Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther 9(4):R74
Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, Kothari M, Lu Y, Fye K, Zhao S, Genant HK (2004) Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 12(3):177–190
Regatte RR, Akella SV, Borthakur A, Kneeland JB, Reddy R (2002) Proteoglycan depletion-induced changes in transverse relaxation maps of cartilage: comparison of T2 and T1rho. Acad Radiol 9(12):1388–1394
Riegger-Krugh C, Gerhart TN, Powers WR, Hayes WC (1998) Tibiofemoral contact pressures in degenerative joint disease. Clin Orthop Relat Res 348:233–245
Sharma L, Eckstein F, Song J, Guermazi A, Prasad P, Kapoor D, Cahue S, Marshall M, Hudelmaier M, Dunlop D (2008) Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum 58(6):1716–1726
Soh L, Tsatsoulis C (1999) Texture analysis of SAR sea ice imagery using gray level co-occurrence matrices. IEEE Trans Geosci Remote Sens 37(2):780–795
Souza RB, Baum T, Wu S, Feeley BT, Kadel N, Li X, Link TM, Majumdar S (2012) Effects of unloading on knee articular cartilage T1rho and T2 magnetic resonance imaging relaxation times: a case series. J Orthop Sports Phys Ther 42(6):511–520
Stehling C, Baum T, Mueller-Hoecker C, Liebl H, Carballido-Gamio J, Joseph GB, Majumdar S, Link TM (2011) A novel fast knee cartilage segmentation technique for T2 measurements at MR imaging—data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 19(8):984–989
Stehling C, Lane NE, Nevitt MC, Lynch J, McCulloch CE, Link TM (2010) Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3T MRI: analysis of a non-symptomatic cohort of the osteoarthritis initiative. Osteoarthritis Cartilage 18(6):776–786
Vasara AI, Jurvelin JS, Peterson L, Kiviranta I (2005) Arthroscopic cartilage indentation and cartilage lesions of anterior cruciate ligament–deficient knees. Am J Sports Med 33(3):408–414
Watrin-Pinzano A, Ruaud JP, Olivier P, Grossin L, Gonord P, Blum A, Netter P, Guillot G, Gillet P, Loeuille D (2005) Effect of proteoglycan depletion on T2 mapping in rat patellar cartilage. Radiology 234(1):162–170
Wayne JS, Kraft KA, Shields KJ, Yin C, Owen JR, Disler DG (2003) MR imaging of normal and matrix-depleted cartilage: correlation with biomechanical function and biochemical composition. Radiology 228(2):493–499
Welsch GH, Mamisch TC, Domayer SE, Dorotka R, Kutscha-Lissberg F, Marlovits S, White LM, Trattnig S (2008) Cartilage T2 assessment at 3-T MR imaging: in vivo differentiation of normal hyaline cartilage from reparative tissue after two cartilage repair procedures–initial experience. Radiology 247(1):154–161
Xia Y, Moody JB, Alhadlaq H (2002) Orientational dependence of T2 relaxation in articular cartilage: a microscopic MRI (microMRI) study. Magn Reson Med 48(3):460–469
Zarins ZA, Bolbos RI, Pialat JB, Link TM, Li X, Souza RB, Majumdar S (2010) Cartilage and meniscus assessment using T1rho and T2 measurements in healthy subjects and patients with osteoarthritis. Osteoarthritis Cartilage 18(11):1408–1416
Acknowledgments
The authors would like to gratefully acknowledge Bill Brock for his time and expertise in acquiring the MR images. This research was funded by Siemens Medical Solutions, USA. The funding source did not contribute to the study design, collection, or interpretation of the data. The funding source did supply the algorithm for calculating T2 values which was their only contribution to the data analysis.
Disclaimer
The Steadman-Philippon Research Institute is a 501(c)(3) non-profit institution supported financially by private donations and corporate support from the following entities: Smith & Nephew Endoscopy, Inc., Arthrex, Inc., Siemens Medical Solutions USA, Inc., ConMed Linvatec, Inc., Össur Americas, Inc., Small Bone Innovations, Inc., Opedix, Inc., Evidence Based Apparel, and Sonoma Orthopedics, Inc.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
See Table 4.
Rights and permissions
About this article
Cite this article
Surowiec, R.K., Lucas, E.P., Fitzcharles, E.K. et al. T2 values of articular cartilage in clinically relevant subregions of the asymptomatic knee. Knee Surg Sports Traumatol Arthrosc 22, 1404–1414 (2014). https://doi.org/10.1007/s00167-013-2779-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-013-2779-2