Abstract
Purpose
A lot of studies on the effect of intra-articular injections are clinical, but many questions on the effect of lidocaine to articular chondrocytes remain unanswered. This study was performed to determine the effects of varying concentrations and exposure times of lidocaine on the viability and proteoglycan metabolism of chondrocytes in vitro.
Method
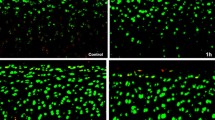
Cartilage was obtained from metatarsal joints of adult bovines. Chondrocytes in alginate beads were cultured in medium containing 6% fetal calf serum at 370 mOsmol at cell densities of 4 million cells/ml. They were then cultured for 24 h under 21% oxygen with 0.125, 0.25, 0.5, and 1% lidocaine and without lidocaine as control. The cell viability profile across intact beads was determined by manual counting using fluorescent probes and transmission electron microscopy.
Result
Lactate production was measured enzymatically as a marker of energy metabolism. Glycosaminoglycan (GAG) accumulation was measured using a modified dimethylmethylene blue assay. Cell viability decreased in a time- and dose-dependent manner in the concentration range of 0.125–1.0% lidocaine under the confocal microscope. Under the electron microscope, apoptosis increased as the concentration of lidocaine increased. GAG accumulation/tissue volume decreases as the concentration of lidocaine increased. However, GAG produced per million cells and the rate of lactate production per live cell were significantly higher for cells cultured at 0.5 and 1% lidocaine than the control group. Bovine chondrocytes cultured in alginate beads under high oxygen pressure are negatively influenced by increasing concentrations of lidocaine.
Conclusion
Cell viability and proteoglycan production (GAG accumulation/tissue volume) decreased as the concentration of lidocaine increased. These data suggest caution in prolonged exposure of cartilage to high concentration lidocaine. Repeated joint injection of lidocaine potentially worsens osteoarthrosis by accelerating cartilage degradation.
Level of evidence
Therapeutic studies—investigating the results of treatment, Level III.





Similar content being viewed by others
References
Bojescul JA, Wilson G, Taylor DC (2005) Idiopathic chondrolysis of the ankle. Arthroscopy 21:224–227
Chiang N, Schwab JM, Fredman G et al (2008) Anesthetics impact the resolution of inflammation. PloS ONE 3:e1879
Chu CR, Izzo NJ, Coyle CH et al (2008) The in vivo effects of bupivacaine on articular chondrocytes. J Bone Joint Surg 90B:814–820
Chunekamrai S, Krook LP, Lust G et al (1989) Changes in articular cartilage after intra-articular injections of methylprednisolone acetate into equine joints. Am J Vet Res 50:1733–1741
Dogan N, Erdem AF, Erman Z et al (2004) The effects of bupivacaine and neostigmine on articular cartilage and synovium in the rabbit knee joint. J Int Med Res 32:513–519
Dragoo JL, Korotkova T, Kanwar R et al (2008) The effect of local anesthetics administered via pain pump on chondrocyte viability. Am J Sports Med 36:1484–1488
Enobakhare BO, Bader DL, Lee DA (1996) Quantification of sulfated glycosaminoglycans in chondrocyte/alginate culture, by use of 1,9-dimethylmethylene blue. Anal Biochem 243:189–191
Foster AH, Carlson BM (1980) Mycotoxicity of local anesthetics and regeneration of the damaged muscle fibers. Anesth Analg 59:727–736
Friederich P, Schmitz TP (2002) Lidocaine-induced cell death in a human model of neuronal apoptosis. Eur J Anaesthesiol 19:564–570
Gray M, Pizzanelli A, Grodzinsky A et al (1988) Mechanical and physiochemical determinants of the chondrocyte biosynthetic response. J Orthop Res 6:777–792
Grimshaw MJ, Mason RM (2000) Bovine articular chondrocyte function in vitro depends upon oxygen tension. Osteoarthr Cartil 8:386–392
Grouselle M, Tueux O, Dabadie P et al (1990) Effect of local anaesthetics on mitochondrial membrane potential in living cells. Biochem J 271:269–272
Guo JF, Jourdian GW, Maccallu DK (1989) Culture and growth characteristics of chondrocytes encapsulated in alginate beads. Connect Tissue Res 19:277–297
Hansen BP, Beck CL, Beck EP et al (2007) Postarthoroscopic glenohumeral chondrolysis. Am J Sports Med 35:1628–1634
Karpie JC, Chu CR (2007) Lidocaine exhibits dose- and time- dependent cytotoxic effects on bovine articular chondrocytes in vitro. Am J Sports Med 35:1621–1627
Kellner K, Liebsch G, Klimant I et al (2002) Determination of oxygen gradients in engineered tissue using a fluorescent sensor. Biotechnol Bioeng 80:73–83
Kobayashi S, Meir A, Urban J (2008) The effect of cell density on the rate of glycosaminoglycan accumulation by disc and cartilage cells in vitro. J Orthop Res 26:493–503
Lee RB, Urban JP (1997) Evidence for a negative Pasteur effect in articular cartilage. Biochem J 321:95–102
Lo IK, Sciore P, Chung M et al (2009) Local anesthetics induce chondrocyte death in bovine articular cartilage disks in a dose- and duration-dependent manner. Arthroscopy 25:707–715
Malda J, Rouwkema J, Martens DE et al (2004) Oxygen gradients in tissue-engineered PEGT/PBT cartilaginous constructs: measurement and modeling. Biotechnol Bioeng 86:9–18
Manjo G, Joris I (1995) Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol 146:3–15
McArthur SD, Gardner DL (1992) Articular cartilage fibrillation and permeability to light green SF dye. A method for the detection of pre-microscopic disease? J Bone Joint Surg 74B:668–672
McCarty DJ (1979) Synovial fluid. In: McCarty DJ (ed) Arthritis and allied conditions. Lea and Febiger, Philadelphia, pp 51–69
Negoro K, Kobayashi S, Taneno K et al (2008) Effect of osmolarity on glycosaminoglycan production and cell metabolism of articular chondrocyte under three dimensional culture system. Clin Exp Rheumatol 26:527–533
Obradovic B, Meldon JH, Freed LE et al (2000) Glycosaminoglycan deposition in engineered cartilage: experiments and mathematical model. AIChE J 46:1860–1871
Orrenius S (2004) Mitochondrial regulation of apoptotic cell death. Toxicol Lett 149:19–23
Petty DH, Jazrawi LM, Estrada LS et al (2004) Glenohumeral chondrolysis after shoulder arthotoscopy: case reports and review of the literature. Am J Sports Med 32:509–515
Piper SL, Kim HT (2008) Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg 90A:986–991
Radwan IA, Saito S, Goto F (2002) The neurotoxicity of local anesthetics on growing neurons: a comparative study of lidocaine, bupivacaine, mepivacaine, and ropivacaine. Anesth Analg 94:319–324
Roughley PJ (2004) Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine 29:2691–2699
Saini S, Wick TM (2003) Concentric cylinder bioreactor for production of tissue engineered cartilage: effect of seeding density and hydrodynamic loading on construct development. Biotechnol Prog 19:510–521
Sanders TG, Zlatkin MB, Paruchuri BN et al (2007) Chondrolysis of the glenohumeral joint after arthroscopy: findings on radiography and low-field-strength MRI. AJR Am J Roentgenol 188:1094–1098
Schumacher HR (2003) Aspiration and injection therapies for joints. Arthritis Rheum 49:413–420
Seshadri V, Coyle CH, Chu CR (2009) Lidocaine potentiates the chondrotoxicity of methylprednisolone. Arthroscopy 25:337–347
Stockwell RA (1991) Cartilage failure in osteoarthritis: relevance of normal structure and function. Clin Anat 4:161–191
Todhunter RJ, Fubini SL, Wootton JA et al (1996) Effect of methylpredonisolone acetate on proteoglycan and collagen metabolism of articular cartilage explants. J Rheumatol 23:1207–1213
Urban JP (1994) The chondrocyte: a cell under pressure. Rheumatology 33:901–908
Venn M, Maroudas A (1977) Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage. I. chemical composition. Ann Rheum 36:121–129
Verzijl N, DeGroot J, Thorpe SR et al (2000) Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem 275:39027–39031
Wilkins RJ, Hall AC (1995) Control of matrix synthesis in isolated bovine chondrocytes by extracellular and intracellular pH. J Cell Physiol 164:474–481
Williams GM, Klein TJ, Sah RL (2005) Cell density alters matrix accumulation in two distinct fractions and the mechanical integrity of alginate-chondrocyte constructs. Acta Biomater 1:625–633
Ysart GE, Mason RM (1994) Responses of articular cartilage explant cultures to different oxygen tensions. Biochim Biophys Acta 1221:15–20
Acknowledgments
The submitted manuscript does not contain information about medical devices or drugs. No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article. We are grateful for the editorial assistance and comments of Dr. Jill Urban of the Department of Physiology, Anatomy and Genetics, Oxford University, UK. This work was supported by Grant-in Aid from the Ministry of Education, Science and Culture of Japan (21591896 and 20791027).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miyazaki, T., Kobayashi, S., Takeno, K. et al. Lidocaine cytotoxicity to the bovine articular chondrocytes in vitro: changes in cell viability and proteoglycan metabolism. Knee Surg Sports Traumatol Arthrosc 19, 1198–1205 (2011). https://doi.org/10.1007/s00167-010-1369-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-010-1369-9