Abstract
Purpose
To test whether a multicomponent intervention would increase the use of low molecular weight heparin (LMWH) over unfractionated heparin (UFH) for venous thromboembolism (VTE) prophylaxis in critically ill patients and change patient outcomes and healthcare utilization.
Methods
Controlled pre–post trial of 12,342 adults admitted to 11 ICUs (five intervention, six control) May 1, 2015 to April 30, 2017 with no contraindication to pharmacological prophylaxis and an ICU stay longer than 24 h. Models were developed to examine temporal changes in ICU VTE prophylaxis (primary outcome), VTE, major bleeding, heparin-induced thrombocytopenia (HIT), death and hospital costs.
Results
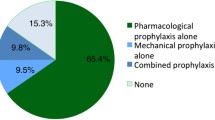
The use of LMWH increased from 45.9% to 78.3% of patient days in the intervention group and from 37.9% to 53.3% in the control group, an absolute increase difference of 17.0% (32.4% vs. 15.4%, p = 0.001). Changes in the administration of UFH were inversely related to those of LMWH. There were no significant differences in the adjusted odds of VTE (ratio of odds ratios [rOR] 1.13, 95% CI 0.51–2.46) or major bleeding (rOR 1.22, 95% CI 0.97–1.54) post-implementation of the intervention (compared to pre-implementation) between the intervention group and the control group. HIT was uncommon in both groups (n = 20 patients). There were no significant changes for ICU and hospital mortality, length of stay and costs. Results were similar when stratified according to reason for ICU admission, patient weight and kidney function.
Conclusions
A multicomponent intervention changed practice, but not clinical and economic outcomes. The benefit of implementing LMWH for VTE prophylaxis under real-world conditions is uncertain.



Similar content being viewed by others
References
Attia J, Ray JG, Cook DJ, Douketis J, Ginsberg JS, Geerts WH (2001) Deep vein thrombosis and its prevention in critically ill adults. Arch Intern Med 161:1268–1279
Protect Investigators for the Canadian Critical Care Trials Group and ANZICSCT Group, Cook D, Meade M, Guyatt G, Walter S, Heels-Ansdell D, Warkentin TE, Zytaruk N, Crowther M, Geerts W, Cooper DJ, Vallance S, Qushmaq I, Rocha M, Berwanger O, Vlahakis NE (2011) Dalteparin versus unfractionated heparin in critically ill patients. N Engl J Med. 364:1305–1314
Fowler RA, Mittmann N, Geerts WH, Heels-Ansdell D, Gould MK, Guyatt G, Krahn M, Finfer S, Pinto R, Chan B, Ormanidhi O, Arabi Y, Qushmaq I, Rocha MG, Dodek P, McIntyre L, Hall R, Ferguson ND, Mehta S, Marshall JC, Doig CJ, Muscedere J, Jacka MJ, Klinger JR, Vlahakis N, Orford N, Seppelt I, Skrobik YK, Sud S, Cade JF, Cooper J, Cook D, Canadian Critical Care Trials Group, Australia and New Zealand Intensive Care Society Clinical Trials Group (2014) Economic evaluation of the prophylaxis for thromboembolism in critical care trial (E-PROTECT): study protocol for a randomized controlled trial. Trials 15:502
Alhazzani W, Lim W, Jaeschke RZ, Murad MH, Cade J, Cook DJ (2013) Heparin thromboprophylaxis in medical-surgical critically ill patients: a systematic review and meta-analysis of randomized trials. Crit Care Med 41:2088–2098
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP (2017) Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 43:304–377
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP (2017) Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 45:486–552
Sauro KM, Bagshaw M, Niven D, Soo A, Brundin-Mather R, Parsons Leigh J, Cook D, Stelfox D (2019) Barriers and facilitators to adopting high value and de-adopting low value practices in the intensive care unit. BMJ Open. https://doi.org/10.1136/bmjopen-2018-024159
Cook D, Duffett M, Lauzier F, Ye C, Dodek P, Paunovic B, Fowler R, Kho ME, Foster D, Stelfox T, Sinuff T, Zytaruk N, Clarke F, Wood G, Cox M, Kutsiogiannis J, Jacka M, Roussos M, Kumar H, Guyatt G, CONECCKT-T (Co-operative Network of Critical Care Knowledge Translation for Thromboprophylaxis) Investigators, Canadian Critical Care Trials Group (2014) Barriers and facilitators of thromboprophylaxis for medical-surgical intensive care unit patients: a multicenter survey. J Crit Care 29(471):e471–e479
Lauzier F, Muscedere J, Deland E, Kutsogiannis DJ, Jacka M, Heels-Ansdell D, Crowther M, Cartin-Ceba R, Cox MJ, Zytaruk N, Foster D, Sinuff T, Clarke F, Thompson P, Hanna S, Cook D, Co-operative Network of Critical Care Knowledge Translation for Thromboprophylaxis Investigators, Canadian Critical Care Trials Group (2014) Thromboprophylaxis patterns and determinants in critically ill patients: a multicenter audit. Crit Care 18:R82
Garcia-Olivares P, Guerrero JE, Galdos P, Carriedo D, Murillo F, Rivera A (2014) PROF-ETEV study: prophylaxis of venous thromboembolic disease in critical care units in Spain. Intensive Care Med 40:1698–1708
Committee on Quality Health Care in America, Institute of Medicine (2001) Crossing the quality chasm: a new health system for the 21st century. National Academy Press, Washington
Niven DJ, Rubenfeld GD, Kramer AA, Stelfox HT (2015) Effect of published scientific evidence on glycemic control in adult intensive care units. JAMA Intern Med 175:801–809
Sinuff T, Muscedere J, Adhikari NK, Stelfox HT, Dodek P, Heyland DK, Rubenfeld GD, Cook DJ, Pinto R, Manoharan V, Currie J, Cahill N, Friedrich JO, Amaral A, Piquette D, Scales DC, Dhanani S, Garland A, Kritical Working Group, Canadian Critical Care Trials Group, Canadian Critical Care Society (2013) Knowledge translation interventions for critically ill patients: a systematic review. Crit Care Med 41:2627–2640
Niven DJ, McCormick TJ, Straus SE, Hemmelgarn BR, Jeffs L, Barnes TRM, Stelfox HT (2018) Reproducibility of clinical research in critical care: a scoping review. BMC Med 16:26
Weiss CH, Krishnan JA, Au DH, Bender BG, Carson SS, Cattamanchi A, Cloutier MM, Cooke CR, Erickson K, George M, Gerald JK, Gerald LB, Goss CH, Gould MK, Hyzy R, Kahn JM, Mittman BS, Moseson EM, Mularski RA, Parthasarathy S, Patel SR, Rand CS, Redeker NS, Reiss TF, Riekert KA, Rubenfeld GD, Tate JA, Wilson KC, Thomson CC, ATS Ad Hoc Committee on Implementation Science (2016) An official American Thoracic Society research statement: implementation science in pulmonary, critical care, and sleep medicine. Am J Respir Crit Care Med 194:1015–1025
Halpern SD, Becker D, Curtis JR, Fowler R, Hyzy R, Kaplan LJ, Rawat N, Sessler CN, Wunsch H, Kahn JM, Choosing Wisely Taskforce, American Thoracic Society, American Association of Critical-Care Nurses, Society of Critical Care Medicine (2014) An official American Thoracic Society/American Association of Critical-Care Nurses/American College of Chest Physicians/Society of Critical Care Medicine policy statement: the choosing Wisely® top 5 list in critical care medicine. Am J Respir Crit Care Med 190:818–826
Niven DJ, Mrklas KJ, Holodinsky JK, Straus SE, Hemmelgarn BR, Jeffs LP, Stelfox HT (2015) Towards understanding the de-adoption of low-value clinical practices: a scoping review. BMC Med 13:255
Des Jarlais DC, Lyles C, Crepaz N, TREND Group (2004) Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health 94:361–366
Pinnock H, Barwick M, Carpenter CR, Eldridge S, Grandes G, Griffiths CJ, Rycroft-Malone J, Meissner P, Murray E, Patel A, Sheikh A, Taylor SJ, StaRI Group (2017) Standards for reporting implementation studies (StaRI) statement. BMJ 356:i6795
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M, Lamb SE, Dixon-Woods M, McCulloch P, Wyatt JC, Chan AW, Michie S (2014) Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 348:g1687
French SD, Green SE, O’Connor DA, McKenzie JE, Francis JJ, Michie S, Buchbinder R, Schattner P, Spike N, Grimshaw JM (2012) Developing theory-informed behaviour change interventions to implement evidence into practice: a systematic approach using the theoretical domains framework. Implement Sci 7:38
Medical Research Council (2000) A framework for the development and evaluation of RCTs for complex interventions to improve health. MRC, London
Sauro KM, Brundin-Mather R, Parsons Leigh J, Niven DJ, Kushner B, Soo A, Cook DJ, Straus S, Doig CJ, Bagshaw S, Stelfox HT (2018) Improving the adoption of optimal venous thromboembolism prophylaxis in critically ill patients: a process evaluation of a complex quality improvement initiative. J Crit Care 50:111–117
Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D (2002) Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 27:299–309
Nyquist P, Bautista C, Jichici D, Burns J, Chhangani S, DeFilippis M, Goldenberg FD, Kim K, Liu-DeRyke X, Mack W, Meyer K (2016) Prophylaxis of venous thrombosis in neurocritical care patients: an evidence-based guideline: a statement for healthcare professionals from the Neurocritical Care Society. Neurocrit Care 24:47–60
Toker S, Hak DJ, Morgan SJ (2011) Deep vein thrombosis prophylaxis in trauma patients. Thrombosis 2011:505373
Fang MC, Fan D, Sung SH, Witt DM, Schmelzer JR, Steinhubl SR, Yale SH, Go AS (2017) Validity of using inpatient and outpatient administrative codes to identify acute venous thromboembolism: the CVRN VTE study. Med Care 55:e137–e143
Sauro KM, Soo A, Kramer A, Couillard P, Kromm J, Zygun D, Niven DJ, Bagshaw SM, Stelfox HT (2018) Venous thromboembolism prophylaxis in neurocritical care patients: are current practices, best practices? Neurocrit Care. https://doi.org/10.1007/s12028-018-0614-9
Kaatz S, Ahmad D, Spyropoulos AC, Schulman S, Subcommittee on Control of Anticoagulation (2015) Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost 13:2119–2126
Chiasson TC, Manns BJ, Stelfox HT (2009) An economic evaluation of venous thromboembolism prophylaxis strategies in critically ill trauma patients at risk of bleeding. PLoS Med 6:e1000098
Drummond M, Sculpher M, Torrance G (2005) Cost analysis. Methods for the economic evaluation of health care programmes. Oxford University Press, Oxford
Agency for Healthcare Research and Quality (2014) Registries for evaluating patient outcomes: a user’s guide. Agency for Healthcare Research and Quality, Rockville
Stelfox HT, Soo A, Niven DJ, Fiest KM, Wunsch H, Rowan KM, Bagshaw SM (2018) Assessment of the safety of discharging select patients directly home from the intensive care unit: a multicenter population-based cohort study. JAMA Intern Med 178:1390–1399
Brundin-Mather R, Soo A, Zuege DJ, Niven DJ, Fiest K, Doig CJ, Zygun D, Boyd JM, Parsons Leigh J, Bagshaw SM, Stelfox HT (2018) Secondary EMR data for quality improvement and research: a comparison of manual and electronic data collection from an integrated critical care electronic medical record system. J Crit Care 47:295–301
Mancl LA, DeRouen TA (2001) A covariance estimator for GEE with improved small-sample properties. Biometrics 57:126–134
Wang M (2015) geesmv: Modified Variance Estimators for Generalized Estimating Equations. R package version 1.3. https://CRAN.R-project.org/package=geesmv
Stelfox HT, Bastos J, Niven DJ, Bagshaw SM, Turin TC, Gao S (2016) Critical care transition programs and the risk of readmission or death after discharge from ICU. Intensive Care Med 42:401–410
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Højsgaard S, Halekoh U, Yan J (2006) The R package geepack for generalized estimating equations. J Stat Softw 15:1–11
Stelfox HT, Niven DJ, Clement FM, Bagshaw SM, Cook DJ, McKenzie E, Potestio ML, Doig CJ, O’Neill B, Zygun D, Critical Care Strategic Clinical Network, Alberta Health Services (2015) Stakeholder engagement to identify priorities for improving the quality and value of critical care. PLoS One 10:e0140141
Gill M, Bagshaw SM, McKenzie E, Oxland P, Oswell D, Boulton D, Niven DJ, Potestio ML, Shklarov S, Marlett N, Stelfox HT, Critical Care Strategic Clinical Network (2016) Patient and family member-led research in the intensive care unit: a novel approach to patient-centered research. PLoS One 11:e0160947
McKenzie E, Potestio ML, Boyd JM, Niven DJ, Brundin-Mather R, Bagshaw SM, Stelfox HT, Improving Daily Care in the ICU Panel (2017) Reconciling patient and provider priorities for improving the care of critically ill patients: a consensus method and qualitative analysis of decision making. Health Expect 20:1367–1374
Kahn SR, Lim W, Dunn AS, Cushman M, Dentali F, Akl EA, Cook DJ, Balekian AA, Klein RC, Le H, Schulman S, Murad MH (2012) Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 141:e195S–e226S
Kahn SR, Morrison DR, Diendere G, Piche A, Filion KB, Klil-Drori AJ, Douketis JD, Emed J, Roussin A, Tagalakis V, Morris M, Geerts W (2018) Interventions for implementation of thromboprophylaxis in hospitalized patients at risk for venous thromboembolism. Cochrane Database Syst Rev 4:CD008201
Writing Group for the C-ICUI, the Brazilian Research in Intensive Care Network, Cavalcanti AB, Bozza FA, Machado FR, Salluh JI, Campagnucci VP, Vendramim P, Guimaraes HP, Normilio-Silva K, Damiani LP, Romano E, Carrara F, Lubarino Diniz de Souza J, Silva AR, Ramos GV, Teixeira C, Brandao da Silva N, Chang CC, Angus DC, Berwanger O (2016) Effect of a quality improvement intervention with daily round checklists, goal setting, and clinician prompting on mortality of critically ill patients: a randomized clinical trial. JAMA 315:1480–1490
Pagoto SL, Lemon SC (2013) Efficacy vs effectiveness. JAMA Intern Med 173:1262–1263
Buchman TG, Azoulay E (2017) Practice guidelines as implementation science: the journal editors’ perspective. Intensive Care Med 43:378–379
Buchman TG, Azoulay E (2017) Practice guidelines as implementation science: the journal editors’ perspective. Crit Care Med 45:553–554
Fretheim A, Soumerai SB, Zhang F, Oxman AD, Ross-Degnan D (2013) Interrupted time-series analysis yielded an effect estimate concordant with the cluster-randomized controlled trial result. J Clin Epidemiol 66:883–887
Acknowledgements
The study was funded by a PRIHS Alberta Innovates—Health Solutions (20100368). HTS was supported by a Population Health Investigator Award from Alberta Innovates—Health Solutions and an Embedded Clinician Researcher Award from the Canadian Institutes of Health Research. SMB was supported by a Canada Research Chair in Critical Care Nephrology and Clinical Investigator Award from Alberta Innovates—Health Solutions. Funding sources had no role in the design, conduct, or reporting of this study and we are unaware of any conflicts of interest. Drs Stelfox and Soo had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Funding sources had no role in the design, conduct, or reporting of this study and we are unaware of any conflicts of interest.
Ethical approval
The health research ethics boards at the University of Calgary (16-0541) and University of Alberta (Pro000065343) approved this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stelfox, H.T., Brundin-Mather, R., Soo, A. et al. A multicentre controlled pre–post trial of an implementation science intervention to improve venous thromboembolism prophylaxis in critically ill patients. Intensive Care Med 45, 211–222 (2019). https://doi.org/10.1007/s00134-019-05532-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-019-05532-1