Abstract
Purpose
To investigate the clinical significance of infection-related ventilator-associated complications (IVAC) and their impact on carbapenem consumption in mechanically ventilated (MV) patients colonised with extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBLE).
Methods
Inception cohort study from the French prospective multicenter OUTCOMEREA database (17 ICUs, 1997–2015) including all ESBLE carriers (systematic rectal swabbing at admission then weekly and/or urinary or superficial surgical site colonisation) with MV duration > 48 h and ≥ 1 episode of IVAC after carriage documentation. All ICU-acquired infections were microbiologically documented.
Results
The 318 enrolled ESBLE carriers (median age 68 years; males 67%; medical admission 68%; imported carriage 53%) experienced a total of 576 IVAC comprising 361 episodes (63%) without documented infection, 124 (21%) related to infections other than ventilator-associated pneumonia (VAP), 73 (13%) related to non-ESBLE VAP and 18 (3%) related to ESBLE VAP. Overall, ESBLE infections accounted for only 43 episodes (7%). Carbapenem exposure within the preceding 3 days was the sole independent predictor of ESBLE infection as the causative event of IVAC, with a protective effect (adjusted odds ratio 0.2, 95% confidence interval 0.05–0.6; P < 0.01). Carbapenems were initiated in 9% of IVAC without infection, 15% of IVAC related to non-VAP infections, 42% of IVAC related to non-ESBLE VAP, and 56% of IVAC related to ESBLE VAP (ESBLE VAP versus non-ESBLE VAP: P = 0.43).
Conclusions
IVAC in ESBLE carriers mostly reflect noninfectious events but act as a strong driver of empirical carbapenem consumption. ESBLE infections are scarce yet hard to predict, strengthening the need for novel diagnostic approaches and carbapenem-sparing alternatives.
Similar content being viewed by others
IVAC in mechanically ventilated ESBLE carriers mostly reflect non-infectious events but act as a strong driver of empirical carbapenem consumption. |
The lack of reliable predictor of ESBLE infections emphasises the need for novel diagnostic approaches and carbapenem-sparing therapeutic alternatives. |
Introduction
The prevalence of colonisation with extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBLE) has reached critical levels in patients admitted to the intensive care unit (ICU) owing to the successful spread of these pathogens in both healthcare settings and community-based populations [1, 2]. Carriage being the main prerequisite for ICU-acquired ESBLE infections [3], current guidelines advocate considering the colonisation status to heighten the likelihood of adequate empirical coverage in patients with suspected nosocomial sepsis [4]. Yet, this approach results in an excess consumption of carbapenems that could hasten the dissemination of carbapenem-resistant Gram-negative bacteria in the hospital environment [5,6,7,8], making the prediction of ESBLE infections a pivotal component of carbapenem-sparing initiatives in identified carriers. This appears especially relevant for patients under mechanical ventilation (MV), with ventilator-associated pneumonia (VAP) accounting for most of suspected and confirmed ICU-acquired infections in this population [9, 10].
A novel algorithm for reporting ventilator-associated events was issued by the Centers for Disease Control and Prevention (CDC) in 2013 [11]. This includes (1) ventilator-associated conditions (VAC)—an episode of worsening oxygenation defined by an increase in required levels of positive-end expiratory pressure (PEEP) and/or FiO2 for at least two calendar days following a period of stability or improvement, (2) infection-related ventilator-associated complications (IVAC)—that is, a subset of VAC with systemic inflammatory response elements suggestive of a new infection and triggering either the initiation of an antimicrobial regimen or the broadening of spectrum in patients already receiving antibiotics, and (3) VAP, which are defined according to this new classification as IVAC with clinical and/or microbiological arguments for pneumonia, regardless of chest radiograph patterns.
Our group and others have previously shown that IVAC are common in MV patients and may be associated with pulmonary or non-pulmonary infections as well as a large panel of sepsis-mimicking conditions not resulting from an infectious event [12,13,14]. However, the epidemiology and clinical significance of IVAC have not been specifically investigated in MV patients colonised with ESBLE. In this inception cohort study, we sought to appraise the causes of IVAC—with a focus on VAP and other ICU-acquired infections due to ESBLE—and their impact on carbapenem exposure in a multicentre population of critically ill ESBLE carriers.
The results of this work were partly presented at the 2017 annual conference of the European Society of Intensive Care Medicine (Vienna, Austria, 23–27 September 2017, abstract 0944) [15].
Patients and methods
Patient data source: the OUTCOMEREA prospective database
This observational study was conducted using a multicentre longitudinal database fuelled since November 1996 by 17 ICUs contributing to the OUTCOMEREA network. The methodology implemented for data collection and quality control has been described elsewhere [5]. Briefly, a minimum of 50 patients older than 16 years and with an ICU stay of more than 24 h are included every year by each participating ICU, which may enter into the database either all admitted patients or only a subset of them following a fixed algorithm that ensures random recruitment (e.g. all admissions in predefined rooms in a given ICU) (Table S1 in the Electronic Supplementary Material, ESM). Data are collected at admission (demographical characteristics, chronic diseases, admission features, baseline severity indexes, admission diagnosis and admission type), then exhaustively recorded on a daily basis throughout the ICU stay [clinical and biological parameters, assessment of organ functions, requirement for MV with levels of PEEP and FiO2, invasive procedures other than MV, fluid challenges, in-hospital transport, iatrogenic events, carriage of multidrug-resistant pathogens, ICU-acquired infections, antibiotic exposure, length of stay (LOS), final diagnoses of the ICU stay, decision to withhold or withdraw life-sustaining therapies, and vital status at ICU and hospital discharge]. These data are prospectively entered into the database by ICU staff through an anonymised electronic case report form using the Vigirea, Rhea and e-Rhea software (OutcomeRea, Rosny-sous-Bois, France). The database protocol was submitted to the Institutional Review Board of the Clermont-Ferrand University hospital (Clermont-Ferrand, France), who waived the need for informed consent (IRB no. 5891). The OUTCOMEREA database has been registered at the Commission Nationale de l’Informatique et des Libertés (registration no. 8999262), in compliance with French law on electronic data sources.
Study population and definitions
Patients admitted between May 1997 and December 2015 were considered for enrolment in the study cohort. We included all ESBLE carriers receiving invasive MV for more than 2 days and with no ESBLE VAP before and at least one episode of IVAC after the documentation of colonisation. Intestinal carriage of ESBLE was universally screened in all participating units by rectal swabbing at ICU admission and weekly afterwards. ESBLE carriage was defined as a positive rectal swab, a positive urine culture without evidence of urinary tract infection or a positive superficial sample from a surgical wound. Of note, colonisation of the lower respiratory tract or the normal skin by multidrug-resistant pathogens—including ESBLE—is not routinely monitored in ICUs contributing to the OUTCOMEREA database. ESBLE carriage was deemed imported in patients with a first positive sample within 48 h following admission and acquired in the ICU in cases of negative admission samples. Patients were considered colonised from the first positive carriage sample to ICU discharge or death.
VAP was diagnosed using standard criteria [16], i.e. new or persistent pulmonary infiltrates on chest X-ray combined with purulent tracheal secretions and/or fever or hypothermia (body temperature greater ≥ 38.5 or ≤ 36.5 °C, respectively) and/or leukocytosis or leukopenia (white blood cells count ≥ 10 × 10.9 or ≤ 4 × 10.9/L, respectively). A definite diagnosis of VAP required microbiological confirmation by quantitative culture from a protected brush [≥ 10.3 colony-forming unit (CFU)/mL], plugged telescopic catheter (≥ 10.3 CFU/mL), bronchoalveolar lavage fluid (≥ 10.4 CFU/mL), or endotracheal aspirate (≥ 10.5 CFU/mL). Other ICU-acquired infections were diagnosed using standard criteria, with microbiological documentation for all cases.
IVAC episodes were retrospectively ascertained by applying the CDC’s definition (see ESM) [11]. We reviewed each episode to identify those associated with VAP, other ICU-acquired infections and non-infectious events within 2 calendar days (i.e. from 8:00 a.m. to 8:00 a.m. the following day) before and after the onset of worsening oxygenation, except for transports and fluid challenges which were only screened within the 2 preceding days. When two non-VAP-associated IVAC occurred within 4 days or less, we only retained the first episode for analyses. Also, IVAC episodes occurring within 8 days after a VAP-associated IVAC were discarded. Lastly, in patients with more than five IVAC, only the first five IVAC were considered to exclude very late episodes in patients with extremely long MV duration. Selected episodes were pooled in four mutually exclusive groups according to causative events, namely IVAC without documented infection (including those only attributable to one or more non-infectious sepsis-mimicking events and those with no identifiable source of infection), non-VAP ICU-acquired infections (involving ESBLE and/or other pathogens), non-ESBLE VAP (with or without a concomitant non-VAP ESBLE infection), and ESBLE VAP.
Statistical analyses
Data are expressed as median [interquartile range (IQR)] for continuous variables and number (%) for categorical variables. Patient characteristics were compared using the Kruskal–Wallis test for continuous variables and the Fisher exact test or χ2 test for categorical variables, as appropriate. Exposure to each antimicrobial class before and after IVAC episodes was compared across the four IVAC groups using the Cochran–Mantel–Haenzel exact test for categorical variables and Cochran–Mantel–Haenzel or Wilcoxon non-parametric tests for continuous variables, with stratification on episode ranks. For these comparisons, β-lactams were pooled according to the classification of Weiss et al. [17].
Factors associated with the identification of an ESBLE infection (VAP or other than VAP) as the causative event of IVAC were first investigated by univariate logistic regression analyses using a marginal model, with each episode being handled independently (generalised estimating equations accounting for unknown covariance structure between distinct episodes in a given patient). Variables yielding P values < 0.20 were then entered into a multiple logistic regression model for the measurement of odds ratios (OR) and 95% confidence intervals (CI), with a diagnosis of IVAC related to an ESBLE infection as the primary outcome. The same procedure was applied to identify factors associated with a diagnosis of ESBLE VAP as the causative event in VAP-related episodes of IVAC. The Akaike information criterion was used in both models to avoid over-fitting. Missing values were handled by single imputation.
All statistical analyses were carried out with SAS v.9.4 (SAS Institute, Cary, NC, USA). P values < 0.05 were considered significant.
Results
Study cohort
We included 318 ESBLE carriers in the study [median age, 67.8 (58.4–76.5) years; males, 66.7%; medical admission, 67.9%] (Fig. 1; Table 1). ESBLE carriage was imported in 169 patients (53.1%) and acquired in the ICU after a median ICU LOS of 11 [7–17] days in the remaining 149 patients (46.9%). Escherichia coli (n = 118, 37.1%), Klebsiella pneumoniae (n = 70, 22%) and Enterobacter spp. (n = 43, 13.5%) accounted for most of the carriage isolates of ESBLE.
Study flow-chart. Numbers of IVAC per patient categories are indicated as median [interquartile range]. ICU intensive care unit, ESBLE extended-spectrum β-lactamase-producing Enterobacteriaceae, MV mechanical ventilation, IVAC infection-related ventilator-associated complication, VAP ventilator-associated pneumonia
Description of IVAC episodes
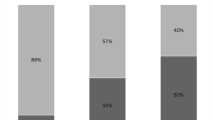
A total of 576 episodes of IVAC were analysed. Among them, 361 episodes (62.7%) were not attributable to a documented ICU-acquired infection, 124 (21.5%) were related to one or more non-VAP ICU-acquired infections, 73 (12.7%) were related to a non-ESBLE VAP, and only 18 (3.1%) were related to an ESBLE VAP (Fig. 2; Table S2 in the ESM). Two or more underlying events were identified in 121 episodes (21%) (Table S2). Overall, only 43 episodes of IVAC (7.5%) were associated with one or more ICU-acquired ESBLE infections (VAP and/or infections other than VAP). Non-VAP ESBLE infections were catheter-related infections with or without bloodstream infection (n = 18), non-catheter-related bloodstream infections (n = 12) and surgical site infections (n = 18).
Causes of IVAC, proportion of episodes attributable to one or more ICU-acquired ESBLE infections and initiation of carbapenem therapy in mechanically ventilated ESBLE carriers. IVAC infection-related ventilator-associated complication, ICU intensive care unit, ESBLE extended-spectrum β-lactamase-producing Enterobacteriaceae, VAP ventilator-associated pneumonia, NS non-significant. The rates of new carbapenem treatments are indicated as measured values and 95% confidence intervals. *P < 0.001 for overall comparison between the four causal groups (no significant difference between non-ESBLE VAP and ESBLE VAP, P = 0.43)
The distribution of IVAC causes—including ESBLE infections—was neither significantly influenced by episode ranking nor by the number of days spent in the ICU since admission or carriage documentation (Table S2). Likewise, the causes of IVAC were similar between patients with imported and ICU-acquired carriage, as between those colonised with ESBL-producing E. coli and other ESBLE species (Tables S3 and S4, respectively).
Predictors of ESBLE infection as the causative event of IVAC
The characteristics of IVAC episodes related to an ESBLE infection (pooled, n = 43) are compared to those of other episodes (n = 533) in Table S5, with corresponding univariate OR. In the multivariable logistic regression model, the sole independent predictor of ESBLE infection was an exposure to carbapenems within the 3 days preceding the occurrence of IVAC, which exerted a protective effect (OR 0.2, 95% CI 0.05–0.6, P < 0.01) (Table 2). This 3-day cut-off correlated with the significant variations that we observed in the frequency of carbapenem pre-exposure across the four IVAC sub-groups on the day preceding the occurrence of IVAC (that is, Day 1) but also from Day 3 to Day 2 before IVAC (Table 3). It is noteworthy that a recent exposure to non-anti-pseudomonal third-generation cephalosporins was not an independent predictor of ESBLE infection (Table S6).
A total of 120 VAP were diagnosed in 93 patients, including 24 ESBLE VAP (20%) in 20 patients (Table S7). In the subset of VAP-related IVAC, no independent predictor of ESBLE VAP could be identified by multivariable logistic regression analysis (Tables S8 and S9).
Correlation between IVAC and antimicrobial exposure
Overall, a new carbapenem-based antimicrobial regimen was started in 94 episodes of IVAC (29.6%) (Table 3). Of note, the empirical use of carbapenems was as frequent in episodes related to an ESBLE VAP as in those resulting from a non-ESBLE VAP (42.5 vs. 55.6%, respectively, P = 0.43). By contrast, an antipseudomonal β-lactam other than a carbapenem (i.e. either cefepime, ceftazidime, aztreonam, ticarcillin-clavulanate or piperacillin-tazobactam) was initiated in only 32 episodes of IVAC (5.5%) (Table S10).
Outcomes
The proportion of patients who died during the episode (overall 18%) and the median subsequent duration of MV [overall, 3 (6–10) days] were higher in VAP-related IVAC than in those with other causes but were similar for IVAC due ESBLE VAP and those due to non-ESBLE VAP (Table S11). Overall in-ICU and in-hospital mortality rates were 33 and 42.1%, respectively (Table 1).
Discussion
In this multicentre cohort of mechanically ventilated ESBLE carriers, a restricted proportion of IVAC (7.5%) resulted from an ICU-acquired ESBLE infection, with VAP accounting for only 3.1% of episodes. No independent predictor of such infections could be identified except the protective effect of a recent or concurrent carbapenem exposure. Strikingly, the empirical initiation of a carbapenem-based regimen was common in IVAC not involving ESBLE.
The prevalence of colonisation with ESBLE is rising steadily in critically ill patients owing to a continuous influx from both community and healthcare ecosystems, with carriage rates at admission currently above 10% in most of ICUs [18,19,20]. This results in an increase in colonisation pressure that may favour subsequent cross-transmission and acquisition during the ICU stay [18, 21,22,23]. Hence, managing an ESBLE carrier with a suspicion of nosocomial infection has become a daily challenge for many intensivists worldwide. ESBLE infections are associated with a high likelihood of inadequate empirical coverage that translates into lower survival rates and extended hospital stays when compared to patients infected with broad-spectrum cephalosporins-susceptible Enterobacteriaceae [5, 24,25,26]. Yet, while negative surveillance samples have a high negative predictive value for ICU-acquired ESBLE infections, the incidence of such infections appears relatively weak in documented carriers (that is, from 10 to 25%), including in those receiving MV [5, 7, 20, 27, 29]. In this work, we attempted an original approach by investigating the clinical significance of IVAC rather than focussing on documented infections in this population. Albeit the accuracy of IVAC as a screening step for VAP remains controversial [16, 28], its definition that combines worsening oxygenation and systemic signs suggestive of a new infection depicts a pragmatic and frequent situation in intubated patients. Our findings indicate that, similarly to what has been reported in the general population of MV patients [12, 13], the occurrence of IVAC in ESBLE carriers correlates with a wide range of healthcare-associated infections as well as non-infectious events, and exerts a cause-dependent prognostic impact, VAP-related IVAC being associated with a worst outcome in terms of subsequent MV duration and short-term mortality than other episodes. More importantly, IVAC secondary to an ESBLE infection were scarce, while most VAP-related episodes implicated pathogens other than ESBLE. These data shed light on the complexity of rationalising the empirical use of carbapenems in ventilated ESBLE carriers when a condition compatible with VAP arises.
Predicting ESBLE infections is pivotal for the fine-tuning of initial therapy in known carriers. The few single-centre studies that addressed this issue yielded conflicting results, notably on the prognostic weight of ESBLE species or prior antimicrobial use [7, 29]. In the present cohort, a carbapenem exposure during the 3 preceding days was the sole feature independently associated with an ESBLE infection as the underlying event of IVAC, with an expected protective effect. We failed to demonstrate any significant association with other potential relevant predictors such as episode rank, invasive procedures, recent exposure to non-carbapenem antimicrobials [particularly β-lactam/β-lactamase inhibitor (BL–BLI) combinations and fluoroquinolones], colonisation with ESBL-producing E. coli versus other ESBLE or imported versus ICU-acquired carriage. Likewise, no independent relationship could be identified when tracking risk factors for ESBLE VAP in the subset of VAP-related IVAC. Thus, no reliable algorithm could be proposed to exclude an ESBLE infection and help restraining the empirical use of carbapenems when IVAC occurs in a documented carrier not recently exposed to this antimicrobial class.
Carbapenems remain the first-line regimen when a severe ESBLE infection is suspected [1, 4, 8]. Here, we observed that these agents were routinely introduced during IVAC not related to an ESBLE infection, especially non-ESBLE VAP. This excessive empirical consumption could lead to deleterious ecological side effects and promote the dissemination of carbapenem-resistant Gram-negative pathogens, at the carrier level as at the hospital scale [6, 7, 30]. Various antimicrobial stewardship initiatives may contribute to reduce the use of these antibiotics. Rapid diagnostic tools, such as direct susceptibility testing or point-of-care molecular assays on clinical samples, stand as promising strategies to narrow the empirical spectrum or allow earlier de-escalation in colonised patients [31,32,33]. Also, a link between the faecal relative abundance of ESBLE and the hazard of subsequent infection has been described in non-ICU patients and warrants further investigations in the specific context of critical illness [34]. In addition, efforts have recently been made to assess the safety of certain BL–BLI combinations as carbapenem-sparing alternatives for both the empirical and definite therapy of ESBLE infections [35]. Evidence has notably emerged to support a role for piperacillin-tazobactam provided that pharmacokinetic parameters are optimised with high-dose regimen and continuous or extended infusion [36, 37]. However, in our work, a recent piperacillin-tazobactam exposure did not protect carriers from ESBLE infections, including VAP. Convincing data are still lacking to appraise the yield of this combination for the initial therapy of severe ESBLE pneumonia [38]. Along this line, new BL–BLI combinations such as ceftolozane-tazobactam and ceftazidime-avibactam have been mainly evaluated in the treatment of complicated UTI or intra-abdominal infections but currently emerge as potential options for the treatment of hospital-acquired pneumonia due to ESBLE, including VAP [39, 40].
Strengths of this study include its multicentre design that minimises the potential influence of local outbreaks on our results, the large number of included patients, the exploitation of prospectively collected data and the use of accurate statistical methods to address its endpoints. Our work also has limitations that deserve to be underlined. First, we did not address the ecological impact of carbapenem misuse in IVAC unrelated to ESBLE infections. Second, the empirical regimens initiated during IVAC were not compared to those of a matched cohort of MV patients not colonised with ESBLE. Third, we did not investigate the potential impact of lower airways colonisation with ESBLE on the causes of IVAC since respiratory surveillance cultures were not routinely performed in participating ICUs. Fourth, all ICU-acquired infections were microbiologically documented, with strict application of validated culture thresholds for the diagnosis of VAP. Hence, we cannot firmly exclude that certain IVAC related to ICU-acquired infections were misclassified as not resulting from an infectious event in patients already receiving appropriate antimicrobial therapy when microbiological samples were collected, thereby over-estimating the protective effect of carbapenem pre-exposure regarding ESBLE infections. Lastly, it remains to be confirmed whether our results may be extrapolated to other critical care environments with distinct colonisation patterns, policies for empirical antimicrobial use and proportion of surgical patients.
In conclusion, the occurrence of IVAC in ESBLE carriers acts as a strong driver of empirical carbapenem use although most of episodes reflect events not resulting from a documented infection and are unrelated to the colonisation status. ESBLE infections appear scarce yet hardly predictable using standard clinical criteria. This study emphasises the global need for novel diagnostic approaches and the validation of carbapenem-sparing empirical regimen in this patient population.
Change history
15 June 2018
The members of the OUTCOMEREA Study Group were provided in such a way that they could not be indexed as collaborators on PubMed. The publisher apologizes for this error and is pleased to list the members of the group here.
References
Bassetti M, De Waele JJ, Eggimann P, Garnacho-Montero J, Kahlmeter G, Menichetti F, Nicolau DP, Paiva JA, Tumbarello M, Welte T, Wilcox M, Zahar JR, Poulakou G (2015) Preventive and therapeutic strategies in critically ill patients with highly resistant bacteria. Intensive Care Med 41(5):776–795
Ruppé E, Woerther PL, Barbier F (2015) Mechanisms of antimicrobial resistance in Gram-negative bacilli. Ann Intensive Care 5:21
Detsis M, Karanika S, Mylonakis E (2017) ICU acquisition rate, risk factors, and clinical significance of digestive tract colonization with extended-spectrum beta-lactamase-producing Enterobacteriaceae: a systematic review and meta-analysis. Crit Care Med 45(4):705–714
Bretonniere C, Leone M, Milesi C, Allaouchiche B, Armand-Lefevre L, Baldesi O, Bouadma L, Decre D, Figueiredo S, Gauzit R, Guery B, Joram N, Jung B, Lasocki S, Lepape A, Lesage F, Pajot O, Philippart F, Souweine B, Tattevin P, Timsit JF, Vialet R, Zahar JR, Misset B, Bedos JP (2015) Strategies to reduce curative antibiotic therapy in intensive care units (adult and paediatric). Intensive Care Med 41(7):1181–1196
Barbier F, Pommier C, Essaied W, Garrouste-Orgeas M, Schwebel C, Ruckly S, Dumenil AS, Lemiale V, Mourvillier B, Clec’h C, Darmon M, Laurent V, Marcotte G, Lucet JC, Souweine B, Zahar JR, Timsit JF (2016) Colonization and infection with extended-spectrum beta-lactamase-producing Enterobacteriaceae in ICU patients: what impact on outcomes and carbapenem exposure? J Antimicrob Chemother 71(4):1088–1097
Armand-Lefevre L, Angebault C, Barbier F, Hamelet E, Defrance G, Ruppé E, Bronchard R, Lepeule R, Lucet JC, El Mniai A, Wolff M, Montravers P, Plesiat P, Andremont A (2013) Emergence of imipenem-resistant Gram-negative bacilli in intestinal flora of intensive care patients. Antimicrob Agents Chemother 57(3):1488–1495
Razazi K, Mekontso Dessap A, Carteaux G, Jansen C, Decousser JW, de Prost N, Brun-Buisson C (2017) Frequency, associated factors and outcome of multi-drug-resistant intensive care unit-acquired pneumonia among patients colonized with extended-spectrum beta-lactamase-producing Enterobacteriaceae. Ann Intensive Care 7(1):61
Gauzit R, Pean Y, Alfandari S, Bru JP, Bedos JP, Rabaud C, Robert J (2015) Carbapenem use in French hospitals: a nationwide survey at the patient level. Int J Antimicrob Agents 46(6):707–712
Bassi GL, Ferrer M, Marti JD, Comaru T, Torres A (2014) Ventilator-associated pneumonia. Semin Respir Crit Care Med 35(4):469–481
Nussenblatt V, Avdic E, Berenholtz S, Daugherty E, Hadhazy E, Lipsett PA, Maragakis LL, Perl TM, Speck K, Swoboda SM, Ziai W, Cosgrove SE (2014) Ventilator-associated pneumonia: overdiagnosis and treatment are common in medical and surgical intensive care units. Infect Control Hosp Epidemiol 35(3):278–284
Magill SS, Klompas M, Balk R, Burns SM, Deutschman CS, Diekema D, Fridkin S, Greene L, Guh A, Gutterman D, Hammer B, Henderson D, Hess D, Hill NS, Horan T, Kollef M, Levy M, Septimus E, Vanantwerpen C, Wright D, Lipsett P (2013) Developing a new, national approach to surveillance for ventilator-associated events: executive summary. Clin Infect Dis 57(12):1742–1746
O’Horo JC, Kashyap R, Sevilla Berrios R, Herasevich V, Sampathkumar P (2016) Differentiating infectious and noninfectious ventilator-associated complications: a new challenge. Am J Infect Control 44(6):661–665
Bouadma L, Sonneville R, Garrouste-Orgeas M, Darmon M, Souweine B, Voiriot G, Kallel H, Schwebel C, Goldgran-Toledano D, Dumenil AS, Argaud L, Ruckly S, Jamali S, Planquette B, Adrie C, Lucet JC, Azoulay E, Timsit JF (2015) Ventilator-associated events: prevalence, outcome, and relationship with ventilator-associated pneumonia. Crit Care Med 43(9):1798–1806
Boyer AF, Schoenberg N, Babcock H, McMullen KM, Micek ST, Kollef MH (2015) A prospective evaluation of ventilator-associated conditions and infection-related ventilator-associated conditions. Chest 147(1):68–81
Barbier F, Bailly S, Schwebel C, Forel JM, Azoulay E, Misset B, Mourvillier B, Reignier J, Darmon M, Zahar JR, Goldgran-Toledano D, de Montmollin E, Souweine B, Timsit JF, OUTCOMEREA Study Group. Infection-related ventilator-associated complications in critically ill patients colonized with extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBLE): correlation with ventilator-associated pneumonia due to ESBLE and impact on carbapenem exposure (abstract #0944). In: Lives 2017—annuel conference of the European Society of Intensive Care Medicine, Vienna, Austria; 2017.
Chastre J, Luyt CE (2016) Does this patient have VAP? Intensive Care Med 42(7):1159–1163
Weiss E, Zahar JR, Lesprit P, Ruppe E, Leone M, Chastre J, Lucet JC, Paugam-Burtz C, Brun-Buisson C, Timsit JF (2015) Elaboration of a consensual definition of de-escalation allowing a ranking of beta-lactams. Clin Microbiol Infect 21(7):649 (e1–10)
Razazi K, Derde LP, Verachten M, Legrand P, Lesprit P, Brun-Buisson C (2012) Clinical impact and risk factors for colonization with extended-spectrum beta-lactamase-producing bacteria in the intensive care unit. Intensive Care Med 38(11):1769–1778
Grohs P, Podglajen I, Guerot E, Bellenfant F, Caumont-Prim A, Kac G, Tillecovidin B, Carbonnelle E, Chatellier G, Meyer G, Fagon JY, Gutmann L (2014) Assessment of five screening strategies for optimal detection of carriers of third-generation cephalosporin-resistant Enterobacteriaceae in intensive care units using daily sampling. Clin Microbiol Infect 20(11):O879–O886
Carbonne H, Le Dorze M, Bourrel AS, Poupet H, Poyart C, Cambau E, Mira JP, Charpentier J, Amarsy R (2017) Relation between presence of extended-spectrum beta-lactamase-producing Enterobacteriaceae in systematic rectal swabs and respiratory tract specimens in ICU patients. Ann Intensive Care 7(1):13
Woerther PL, Burdet C, Chachaty E, Andremont A (2013) Trends in human fecal carriage of extended-spectrum beta-lactamases in the community: toward the globalization of CTX-M. Clin Microbiol Rev 26(4):744–758
Masse J, Elkalioubie A, Blazejewski C, Ledoux G, Wallet F, Poissy J, Preau S, Nseir S (2017) Colonization pressure as a risk factor of ICU-acquired multidrug resistant bacteria: a prospective observational study. Eur J Clin Microbiol Infect Dis 36(5):797–805
Gurieva T, Dautzenberg MJD, Gniadkowski M, Derde LPJ, Bonten MJM, Bootsma MCJ (2017) The transmissibility of antibiotic-resistant Enterobacteriaceae in intensive care units. Clin Infect Dis. https://doi.org/10.1093/cid/cix825
Rottier WC, Ammerlaan HS, Bonten MJM (2012) Effects of confounders and intermediates on the association of bacteraemia caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae and patient outcome: a meta-analysis. J Antimicrob Chemother 67(6):1311–1320
Schwaber MJ, Carmeli Y (2007) Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J Antimicrob Chemother 60(5):913–920
de Kraker ME, Wolkewitz M, Davey PG, Koller W, Berger J, Nagler J, Icket C, Kalenic S, Horvatic J, Seifert H, Kaasch A, Paniara O, Argyropoulou A, Bompola M, Smyth E, Skally M, Raglio A, Dumpis U, Melbarde Kelmere A, Borg M, Xuereb D, Ghita MC, Noble M, Kolman J, Grabljevec S, Turner D, Lansbury L, Grundmann H (2011) Burden of antimicrobial resistance in European hospitals: excess mortality and length of hospital stay associated with bloodstream infections due to Escherichia coli resistant to third-generation cephalosporins. J Antimicrob Chemother 66(2):398–407
Bruyere R, Vigneron C, Bador J, Aho S, Toitot A, Quenot JP, Prin S, Emmanuel Charles P (2016) Significance of prior digestive colonization with extended-spectrum beta-lactamase-producing Enterobacteriaceae in patients with ventilator-associated pneumonia. Crit Care Med 44(4):699–706
Fan Y, Gao F, Wu Y, Zhang J, Zhu M, Xiong L (2016) Does ventilator-associated event surveillance detect ventilator-associated pneumonia in intensive care units? A systematic review and meta-analysis. Crit Care 20(1):338
Vodovar D, Marcadié G, Rousseau H, Raskine L, Vicaut E, Deye N, Baud FJ, Mégarbane B (2014) Predictive factors for extended-spectrum beta-lactamase producing Enterobacteriaceae causing infection among ICU patients with prior colonization. Infection 42(4):743–748
Miliani K, L’Heriteau F, Lacave L, Carbonne A, Astagneau P (2011) Imipenem and ciprofloxacin consumption as factors associated with high incidence rates of resistant Pseudomonas aeruginosa in hospitals in northern France. J Hosp Infect 77(4):343–347
Cohen J, Vincent JL, Adhikari NK, Machado FR, Angus DC, Calandra T, Jaton K, Giulieri S, Delaloye J, Opal S, Tracey K, van der Poll T, Pelfrene E (2015) Sepsis: a roadmap for future research. Lancet Infect Dis 15(5):581–614
Le Dorze M, Gault N, Foucrier A, Ruppe E, Mourvillier B, Woerther PL, Birgand G, Montravers P, Dilly MP, Tubach F, Andremont A, Timsit JF, Wolff M, Armand-Lefevre L (2015) Performance and impact of a rapid method combining mass spectrometry and direct antimicrobial susceptibility testing on treatment adequacy of patients with ventilator-associated pneumonia. Clin Microbiol Infect 21(5):468 (e1–6)
Kollef MH, Burnham CD (2017) Ventilator-associated pneumonia: the role of emerging diagnostic technologies. Semin Respir Crit Care Med 38(3):253–263
Ruppé E, Lixandru B, Cojocaru R, Buke C, Paramythiotou E, Angebault C, Visseaux C, Djuikoue I, Erdem E, Burduniuc O, El Mniai A, Marcel C, Perrier M, Kesteman T, Clermont O, Denamur E, Armand-Lefevre L, Andremont A (2013) Relative fecal abundance of extended-spectrum-beta-lactamase-producing Escherichia coli strains and their occurrence in urinary tract infections in women. Antimicrob Agents Chemother 57(9):4512–4517
Harris PN, Tambyah PA, Paterson DL (2015) Beta-lactam and beta-lactamase inhibitor combinations in the treatment of extended-spectrum beta-lactamase producing Enterobacteriaceae: time for a reappraisal in the era of few antibiotic options? Lancet Infect Dis 15(4):475–485
Gutierrez-Gutierrez B, Perez-Galera S, Salamanca E, de Cueto M, Calbo E, Almirante B, Viale P, Oliver A, Pintado V, Gasch O, Martinez-Martinez L, Pitout J, Akova M, Pena C, Molina J, Hernandez A, Venditti M, Prim N, Origuen J, Bou G, Tacconelli E, Tumbarello M, Hamprecht A, Giamarellou H, Almela M, Perez F, Schwaber MJ, Bermejo J, Lowman W, Hsueh PR, Mora-Rillo M, Natera C, Souli M, Bonomo RA, Carmeli Y, Paterson DL, Pascual A, Rodriguez-Bano J (2016) A multinational, preregistered cohort study of beta-lactam/beta-lactamase inhibitor combinations for treatment of bloodstream infections due to extended-spectrum-beta-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 60(7):4159–4169
Guet-Revillet H, Tomini E, Emirian A, Join-Lambert O, Lecuyer H, Zahar JR, Jullien V (2017) Piperacillin/tazobactam as an alternative antibiotic therapy to carbapenems in the treatment of urinary tract infections due to extended-spectrum beta-lactamase-producing Enterobacteriaceae: an in silico pharmacokinetic study. Int J Antimicrob Agents 49(1):62–66
Timsit JF, Pilmis B, Zahar JR (2017) How should we treat hospital-acquired and ventilator-associated pneumonia caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae? Semin Respir Crit Care Med 38(3):287–300
Torres A, Zhong N, Pachl J, Timsit JF, Kollef M, Chen Z, Song J, Taylor D, Laud PJ, Stone GG, Chow JW (2017) Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): a randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect Dis. https://doi.org/10.1016/s1473-3099(17)30747-8
Wright H, Bonomo RA, Paterson DL (2017) New agents for the treatment of infections with Gram-negative bacteria: restoring the miracle or false dawn? Clin Microbiol Infect 23(10):704–712
Acknowledgements
Members of the OUTCOMEREA Study Group—Scientific Committee: Jean-François Timsit (Medical and Infectious Diseases ICU, Bichat-Claude Bernard Hospital, Paris, France; UMR 1137 Inserm-Paris Diderot university IAME, F75018, Paris); Elie Azoulay (Medical ICU, Saint Louis Hospital, Paris, France); Maïté Garrouste-Orgeas (ICU, Saint-Joseph Hospital, Paris, France); Jean-Ralph Zahar (Infection Control Unit, Angers Hospital, Angers, France); Christophe Adrie (Physiology, Cochin Hospital, Paris, France); Michael Darmon (Medical ICU, Saint Etienne University Hospital, St Etienne, France); and Christophe Clec’h (ICU, Avicenne Hospital, Bobigny, and UMR 1137 Inserm, Paris Diderot university IAME, F75018, Paris, France).
Biostatistical and information system expertise: Jean-Francois Timsit (Medical and Infectious Diseases ICU, Bichat-Claude Bernard Hospital, Paris, France; UMR 1137 Inserm –Paris Diderot university IAME, F75018, Paris); Corinne Alberti (Medical Computer Sciences and Biostatistics Department, Robert Debré Hospital, Paris, France); Adrien Français (Integrated Research Center U823, Grenoble, France); Aurélien Vesin (OUTCOMEREA organization and Integrated Research Center U823, Grenoble, France); Stephane Ruckly (OUTCOMEREA organization and Inserm UMR 1137 IAME, F75018, Paris); Sébastien Bailly (Grenoble university hospital Inserm UMR 1137 IAME, F75018, Paris) and Christophe Clec’h (ICU, Avicenne Hospital, Bobigny, and Inserm UMR 1137 IAME, F75018, Paris, France); Frederik Lecorre (Supelec, France); Didier Nakache (Conservatoire National des Arts et Métiers, Paris, France); and Aurélien Vannieuwenhuyze (Tourcoing, France).
Investigators of the OUTCOMEREA database: Dr Romain HERNU, Christophe Adrie (ICU, CH Melun, and Physiology, Cochin Hospital, Paris, France); Carole Agasse (medical ICU, university hospital Nantes, France); Bernard Allaouchiche (ICU, Pierre benite Hospital, Lyon, France); Olivier Andremont (ICU, Bichat Hospital, Paris, France); Pascal Andreu (CHU Dijon, Dijon, France); Laurent Argaud (Medical ICU, Hospices Civils de Lyon, Lyon, France); Claire Ara-Somohano (Medical ICU, University Hospital, Grenoble, France); Elie Azoulay (Medical ICU, Saint Louis Hospital, Paris, France); François Barbier (medical-surgical ICU, Orleans, France), Déborah Boyer (ICU, CHU Rouen, France), Jean-Pierre Bedos (ICU, Versailles Hospital, Versailles, France); Thomas Baudry (Medial ICU, Edouard Heriot hospital, Lyon France), Jérome Bedel (ICU, Versailles Hospital, Versailles, France), Julien Bohé (ICU, Hôpital Pierre Benite, Lyon France), Lila Bouadma (ICU, Bichat Hospital, Paris, France); Jeremy Bourenne (Réanimation des urgencies, Timone-2; APHM, Marseille, France); Noel Brule (medical ICU, university hospital Nantes, France); Cédric Brétonnière (medical ICU, university hospital Nantes, France); Christine Cheval (ICU, Hyeres Hospital, Hyeres, France); Julien Carvelli (Réanimation des urgencies, Timone-2; APHM, Marseille, France);Christophe Clec’h (ICU, Avicenne Hospital, Bobigny, France); Elisabeth Coupez (ICU, G Montpied Hospital, Clermont-Ferrand, France); Martin Cour Medial ICU, Edouard Heriot hospital, Lyon France), Michael Darmon (ICU, Saint Etienne Hospital, Saint Etienne, France); Etienne de Montmollin (ICU, Delafontaine Hospital, Saint Denis), Loa Dopeux (ICU, G Montpied Hospital, Clermont-Ferrand, France); Anne-Sylvie Dumenil (Antoine Béclère Hospital, Clamart, France); Claire Dupuis (Bichat hospital and UMR 1137 Inserm –Paris Diderot university IAME, F75018, Paris, France), Jean-Marc Forel (AP HM, Medical ICU, Hôpital Nord Marseille), Marc Gainnier (Réanimation des urgencies, Timone-2; APHM, Marseille, France), Charlotte Garret (medical ICU, university hospital Nantes, France); Steven Grangé (ICU, CHU Rouen, France), Antoine Gros (ICU, Versailles Hospital, Versailles, France), Akim Haouache (Surgical ICU, H Mondor Hospital, Creteil, France); Romain Hernu (Medical ICU, Hospices Civils de Lyon, Lyon, France); Tarik Hissem (ICU, Eaubonne, France), Vivien Hon Tua Ha (ICU, CH Meaux, France); Sébastien Jochmans (ICU, CH Melun); Jean-Baptiste Joffredo (ICU, G Montpied Hospital, Clermont-Ferrand, France); Hatem Kallel (ICU, Cayenne General Hospital, Cayenne, France); Guillaume Lacave (ICU, Versailles Hospital, Versailles, France), Alexandre Lautrette (ICU, G Montpied Hospital, Clermont-Ferrand, France); Virgine Lemiale (Medical ICU, Saint Louis Hospital, Paris, France); Mathilde Lermuzeaux (ICU, Bichat Hospital, Paris, France), Guillaume Marcotte (Surgical ICU, Hospices Civils de Lyon, Lyon, France); Jordane Lebut (ICU, Bichat Hospital, Paris, France); Maxime Lugosi (Medical ICU, University Hospital Grenoble, Grenoble, France); Eric Magalhaes (ICU, Bichat Hospital, Paris, France), Sibylle Merceron (ICU, Versailles Hospital, Versailles, France), Bruno Mourvillier (ICU, Bichat Hospital, Paris, France); Benoît Misset (ICU, Saint-Joseph Hospital, Paris, France and Medical ICU CHU Rouen, France); Bruno Mourvillier (ICU, Bichat Hospital, Paris, France); Mathild Neuville (ICU, Bichat Hospital, Paris, France), Laurent Nicolet (medical ICU, university hospital Nantes, France); Johanna Oziel (Medico-surgical ICU, hôpital Avicenne APHP, Bobigny, France), Laurent Papazian (Hopital Nord, Marseille, France), Benjamin Planquette (pulmonology ICU, George Pompidou hospital Hospital, Paris, France); Jean-Pierre Quenot (CHU Dijon, Dijon, France); Aguila Radjou (ICU, Bichat Hospital, Paris, France), Marie Simon (Medial ICU, Edouard Heriot hospital, Lyon France), Romain Sonneville (ICU, Bichat Hospital, Paris, France), Jean Reignier (medical ICU, university hospital Nantes, France); Bertrand Souweine (ICU, G Montpied Hospital, Clermont-Ferrand, France); Carole Schwebel (ICU, A Michallon Hospital, Grenoble, France); Shidasp Siami (ICU, Eaubonne, France); Roland Smonig (ICU, Bichat Hospital, Paris, France); Gilles Troché (ICU, Antoine Béclère Hospital, Clamart, France); Marie Thuong (ICU, Delafontaine Hospital, Saint Denis, France); Guillaume Thierry (ICU, Saint-Louis Hospital, Paris, France); Dany Toledano (ICU, Gonesse Hospital, Gonesse, France); Guillaume Van Der Meersch, Medical Surgical ICU, university hospital Avicenne), Marion Venot (Medical ICU, Saint Louis Hospital, Paris, France); Olivier Zambon (medical ICU, university hospital Nantes, France);
Study Monitors: Julien Fournier, Caroline Tournegros, Stéphanie Bagur, Mireille Adda, Vanessa Vindrieux, Sylvie de la Salle, Pauline Enguerrand, Loic Ferrand, Vincent Gobert, Stéphane Guessens, Helene Merle, Nadira Kaddour, Boris Berthe, Samir Bekkhouche, Kaouttar Mellouk, Mélaine Lebrazic, Carole Ouisse, Diane Maugars, Christelle Aparicio, Igor Theodose, Manal Nouacer, Veronique Deiler, Myriam Moussa, Atika Mouaci, Nassima Viguier and Sophie Letrou.
Funding
OUTCOMEREA and COMBACTE-MAGNET (SB and JFT are supported by the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115737-2—COMBACTE-MAGNET, resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013) and EFPIA companies; JFT was additionally supported by grant no. 115523—COMBACTE-NET).
Author information
Authors and Affiliations
Consortia
Contributions
Study design, data analysis and interpretation of the result: FB, SB, JFT. Data acquisition: CS, LP, EA, HK, SS, LA, GM, BM, JR, MD, JRZ, GDT, EdM, BS, BM. Writing of the article: FB, SB, JFT. Critical revision for important intellectual content: CS, LP, EA, HK, SS, LA, GM, BM, JR, MD, JRZ, GDT, EdM, BS, BM. Approval of the submitted article: all authors.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest relative to the present study.
Additional information
Members of the OUTCOMEREA Study Group are listed in the Acknowledgements.
François Barbier and Sébastien Bailly contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Barbier, F., Bailly, S., Schwebel, C. et al. Infection-related ventilator-associated complications in ICU patients colonised with extended-spectrum β-lactamase-producing Enterobacteriaceae. Intensive Care Med 44, 616–626 (2018). https://doi.org/10.1007/s00134-018-5154-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-018-5154-4