Abstract
Purpose
To describe all post-insertion complications involving most used intravascular access, and to determine whether the use of a new-generation transparent dressing (3M™ IV Advanced) might reduce their number and impact on ICU patient outcomes.
Methods
Patients older than 18, with an expected length of stay ≥48 h and requiring at least one central venous catheter (CVC), arterial catheter (AC), haemodialysis catheter (HDC), pulmonary arterial catheters (PAC) or peripheral venous catheter (PVC) were randomized into two groups: a new-generation transparent dressing, or the hospital’s classical transparent dressing, and were followed daily for any infectious and non-infectious complications. Complications were graduated for severity by an independent international multicentre multidisciplinary panel of practitioners using a Delphi process.
Results
We included 628 patients, 2214 catheters (873 PVCs, 630 CVCs, 512 ACs and 199 HDCs and PACs) and 4836 dressings. Overall incidence rate was of 60.9/1000 catheter-days. The most common complication was dysfunction (34.6/1000 catheter-days), mainly for PVCs (16/1000 catheter-days) and ACs (12.9/1000 catheter-days). Infectious complications incidence rate in CVCs and ACs was of 14.5/1000, mostly due to colonization (14.2/1000 catheter-days). Thrombosis incidence was of 3.8/1000 catheter-days with severe and very severe complications in 16 cases (1.8/1000 catheter-days) and one thrombosis-related death. 3M™ IV Advanced dressing did not decrease the rate of catheters with at least a minor complication [57.37/1000 vs. 57.52/1000 catheter-days, HR 1.03, CI (0.84–1.27), p = 0.81]. Incidence rates for each single complication remained equivalent: infectious [HR 0.93 (0.62–1.40), p = 0.72], deep thrombosis [HR 0.90 (0.39–2.06), p = 0.80], extravasation and phlebitis [HR 1.40 (0.69–2.82), p = 0.35], accidental removal [1.07 (0.56–2.04), p = 0.84] and dysfunction [HR 1.04 (0.80–1.35), p = 0.79].
Conclusion
The ADVANCED study showed the overall risk of complications to intravascular catheters in ICU patients being dysfunction, infection and thrombosis. The 3M™ IV Advanced dressing did not decrease complication rates as compared to standard dressings.
Similar content being viewed by others
Introduction
Intravascular catheters (IVCs) are the most ubiquitous medical devices in hospital, and 28.7 % of in-hospital patients benefit from at least one catheter insertion [1]. In intensive care units (ICUs), where the presence of a high-quality vascular access is essential, the proportion of patients receiving of a catheter insertion rises to up to 88.7 % [1], including a wide variety of devices.
Despite the clear benefits they provide, there is a growing recognition of the eventual risks associated with IVCs. Catheters can fail before the completion of treatment as a result of accidental removal, occlusion, thrombosis or infection, which may result in increased mortality, morbidity and higher ICU length of stay [2–4]. Indeed, catheter-related infection in particular [4–8] and also catheter-associated deep-vein thrombosis [9] are widely studied complications; other adverse events such as catheter failure, accidental removal or superficial thrombosis and extravasations still need further research. Some studies [10, 11] point out that catheter movements within the vein, resulting from a poor securement, have an impact on catheter-related infections, phlebitis and thrombosis; they also suggest that stabilization provided by dressings or securement devices could have a role in dislodgement and catheter restart rates. Technological innovations in catheter materials and dressings allow improvements in safety and efficiency [12], but as highlighted by recent reviews [13, 14], randomized controlled trials demonstrating the benefits of better catheter securement trials in clinical settings need to be performed.
This article reports the results of a prospective randomized controlled trial aimed at describing the different post-insertion complications that affect the most used intravascular devices in ICUs and determining whether the use of a new-generation transparent dressing (3M™ IV Advanced), compared to classical dressings, could help reduce these adverse events.
The aim of the ADVANCED study was twofold: to describe all the post-insertion complications related to intravascular access in intensive care, and to investigate the clinical performance of a new-generation transparent dressing, 3M™ IV Advanced Securement dressing, compared to classical dressings used in a medical ICU from a tertiary hospital in France.
All dressings were compared in terms of efficiency (post-insertion complication rates), effectiveness (indwell time without dressing disruption) and safety (tolerance and patient comfort). Post-insertion complications were divided into infectious complications (colonization and infection), thromboembolic complication (thrombosis and phlebitis) and other complications (catheter dysfunction, extravasation and accidental catheter removal).
Design and methods
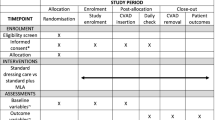
Study design and sample
This prospective, single-centre randomized controlled trial was conducted in an 18-bed medical ICU at a tertiary hospital in France, between October 2012 and October 2013. We included all male and female patients who were at least 18 years old, admitted to the ICU for an expected stay of at least 48 h and requiring a catheter insertion as part of their clinical care. Patients were excluded if they had an existing bloodstream infection, a known hypersensitivity to the study dressings or if they refused their consent.
We calculated a sample size of 670 patients with an average of two catheters per patient. With an overall post-insertion complication rate of 30 %, this size would be sufficient to detect a relative difference of 6 % reduction rate, equivalent to an absolute reduction of 35 % in the number of post-insertion complications.
Eligible patients were randomly assigned on admission via a dedicated computer-generated allocation sequence (random permuted blocks), in a one-to-one ratio, to either group of dressings:
-
(a)
Study group: 3M™ IV Advanced.
-
(b)
Control group: depending on the type of dressing available at the hospital, either 3M™ HP Dressing (1st period, from October 2012 to February 2013) or Smith & Nephew IV3000™ (2nd period, from February 2013 to October 2013).
All consecutive catheters in a given patient were managed as determined by the random allocation until ICU discharge. Patients were followed until catheter removal or discharge from the unit, plus 48 h of follow-up. If adverse reactions occurred, patients were followed up until the incident was fully investigated.
Ethics statement
The study received the approval of the Rhone-Alpes-V Ethics Committee, France, in July 2012 (N ID RCB 2012-A00734-39). Informed consent was sought prior to a patient’s participation or, if their clinical condition meant they were unable to express it, permission from their legal representative was sought and patients were asked retrospectively.
Statistical analysis
Statistical analysis was performed using SAS 9.3 (Cary, NC). Characteristics of patients, catheters and dressings were described using frequency and percentage (qualitative variables) or median and interquartile range (quantitative variables). Balance of characteristics between randomization groups was tested using the Fisher exact test or Mann–Whitney test as appropriate.
The relationship between randomization group and the number of dressings per catheter was assessed using a generalized estimating equation (GEE) with negative binomial distribution to take into account intrapatient correlation between catheters. Adjustments based on time period, insertion duration and type of catheter were systematically applied when appropriate, and to test for group differences concerning first-sight imbalances (i.e. SAPS II), no significant differences were found (Electronic Supplemental Appendix (ESA) 3.2, Table S3). The differences in the incidence of catheter-associated complications between randomization groups were tested using a marginal Cox model. This model takes into account the censored nature of the data and accounts for the intracluster (intrapatient) correlation (>1 catheter per patient), using a robust sandwich variance estimate (PROC PHREG of the SAS software). All P values less than 5 % were considered as significant.
Staff training and intravascular catheter care
Prior to implementation of the study, research staff ensured that all staff members were aware of institutional protocols of catheter insertion, maintenance and surveillance, following the French Haute Autorité de Santé Checklist and international guidelines for preventing catheter-related infections (ESA 2.1: Guidelines for insertion and management of central and peripheral lines used during the study).
Dressings and catheters sites were inspected at each shift by the bedside nurses, and at least daily by a research nurse and a medical investigator; to reduce the subjectivity related to the large number of caregivers involved, training and posters with standard grids and pictures were provided. Dressing effectiveness was based on the rate of unplanned dressing changes, defined as the need for changing the dressing before the time interval specified by the local guidelines.
Follow-up
Arterial and central venous catheters received a chlorhexidine-impregnated sponge on the insertion site and under the transparent dressing, as part of the standard dressing. Dressing replacement interval was 7 days for these devices and every 4 days for all other catheters.
Tip culture was systematically performed for all central and arterial catheters and only in the case of suspected infection for peripheral catheters. Catheter infection and colonization was defined as previously reported according to French guidelines [15] and detailed in the ESA. Definitions for all catheter complications (dysfunction, extravasation, unexpected catheter removal and thromboembolic complications) were established following international and national guidelines, scores and scales (ESA 2.3).
To determine the effect of the dressings on skin tolerance, we evaluated the skin reaction at each dressing change and at each catheter removal according to the International Contact Dermatitis Research Group (ICDRG) scale and on a clinical evaluation of any abnormality such as redness, pain, oedema and purulent or non-purulent discharge.
Materials
All dressings used in the study were transparent, waterproof, impervious to the passage of bacteria to ensure a sterile barrier, and used a hypoallergenic adhesive. The hospital’s standard dressings were 3M™ Tegaderm HP dressing. The production was stopped by 3M after 4 months, and Smith & Nephew™ IV3000 dressing was used during the rest of the study. Differences with the 3M™ IV Advanced Securement dressing concerned the evacuation of the excess moisture to prevent skin maceration, and the adaptability to the anatomical contours.
Graduation of infectious and non-infectious complications by a Delphi process
Concomitantly with the study, and in order to integrate the various scales and classifications in a comparative table, we implemented a Delphi process focused on the impact of complications. On the basis of a literature review and on the NCI Common Terminology Criteria for Adverse Events v4 (CTCAE) (ESA 2.2, Table S1), two investigators (SCG, JFT) developed the Delphi questionnaires. An independent expert panel of 12 doctors and 11 nurses (ESA 1.1), mostly working outside the study centre to ensure higher objectivity, evaluated the severity of complications within each category (infection, thrombosis, accidental removal, extravasation, dysfunction), and that for each study device.
The experts received a first questionnaire listing the different post-insertion complications without particular hierarchy and had to rate each item in terms of severity using a visual scale. Only items that achieved over 80 % of agreement were considered as solved. Discordance between experts was solved after three rounds. In the last round specific discrepancies between classifications were solved by specific comparisons (ESA 1.3). The consensus obtained allowed us to build a summarizing table (Table 4), which even includes the complications that did not appear in the CTCAE classification. Detailed graduation and definitions of the CTCAE items used for this study are in ESA 2.2.
Results
The ADVANCED study included and evaluated 628 patients, 4836 dressings and 2214 catheters: 873 peripheral venous catheters, 630 central venous catheters, 512 arterial catheters and 199 dialysis catheters and pulmonary arterial catheters (Fig. 1). Characteristics of patients are given in Tables S2 and S3 of the ESA. Patients were similar in all the groups in terms of sex, comorbidities, SOFA score and length of stay at the ICU. All analyses were controlled ensuring that the outcome measures were not affected. Characteristics of the catheters are summarized in Table 1. The study did not find differences between the dressings in terms of complication rates, dressing disruption rates or dressing tolerance.
Complication rates
Although from the 684 initially enrolled patients only 628 were finally included, the 2214 evaluable catheters largely exceeded the size calculated for performing the intention-to-treat analysis (1340). Overall complication rate was high, with an incidence density of 60.9/1000 catheter-days, involving 26.9 % of all intravenous devices (Table 2 and ESA 3.1, Table S2). Incidence rates and severity were extremely different, depending on the kind of device and their impact on the patient; 267 complications occurred with PVC (41.9 % of all PVCs; 25.5/1000 catheter-days), severe or very severe complications representing 2 cases (0.4 %). One hundred and eleven (17.6 % of all CVCs; 10.6/1000 catheter-days) of the CVCs presented a complication with 22 (7 %) severe and 4 (3 %) very severe complications. Sixty-one HDCs presented a complication (30.6 % of all HDCs, 5.8/1000 catheter-days), with 34 (17.1 %) severe and 3 (1.5 %) very severe events with life-threatening consequences.
The most frequent complication leading to early catheter removal was dysfunction, with an incidence rate of 34.6/1000 catheter-days; this event occurred mainly with PVCs (16/1000 catheter-days), followed by ACs (12.9/1000 catheter-days) and HDCs (3.5/1000 catheter-days). Overall infectious complications incidence rate was of 14.5/1000 catheter-days and mainly related to catheter-tip colonization (14.2/1000 catheter-days), as only one catheter-related infection (0.1/1000 catheter-days) in an AC and two catheter-related bloodstream infections (0.2/1000 catheter-days), in a CVC and in an HDC, happened during the study.
Thrombosis, deep or superficial, appeared as the most serious complication, with 16 cases of severe thrombosis (1.8/1000 catheter-days), five very severe (0.6/1000 catheter-days) and one attributable death (0.1/1000 catheter-days), and an overall incidence rate of 3.8/1000 catheter-days. Deep-vein thrombosis rates were also higher for HDCs, as it occurred in 5 % of all HDCs and in less than 1 % of ACs or CVCs. For PVCs, extravasation and superficial phlebitis occurred in 45 cases (24.7/1000 catheter-days).
Accidental catheter removal was identified in 71 cases (3.2 % of all catheters, density incidence of 6.7/1000 catheter-days) and occurred mainly with PVCs (55 cases, 6 % of all PVCs, 5.2/1000 catheter-days) and CVCs (14 cases, 2.2 % of all CVCs, 1.3/1000 catheter-days), whereas it only happened on two ACs (1.2/1000 catheter-days) and never affected HDCs or PACs.
Complication rate was identical between groups, with an incidence rate of at least a minor complication of 57.37/1000 catheter-days for the Advanced group and of 57.52 for the control group [HR 1.03, CI (0.84–1.27), p = 0.81], proving the equivalence in performance between the three dressing types (Table 3). At least one minor complication occurred in 20 % versus 16.45 % in period 1 (HR = 1.14, p = 0.56) and in 25.5 % versus 26.6 % in period 2 (HR = 0.95, p = 0.71), but these differences were not significant (Table 4).
Secondary end points: dressing disruption rates and skin tolerance
As dressings of PVCs are generally removed only during catheter replacement, this end point was analysed only for all other catheters: ACs, CVCs, PACs and HDCs. Overall disruption rate was 36.9 %, but not different between both groups (ESA 3.4, Table S5). There was no difference either in the median and mean number of dressings per group (2 [1–3], 2.2 for the Advanced group versus 2 [1–3], 2.1 for the control group, RR = 1.04, p = 0.19) (ESA 3.4, Table S5). The main reason for disruption was a peeling off by the edges, inducing a dressing change to secure the catheter and seal the insertion site (ESA 3.5, Table S6 and ESA 3.6, Table S7].
All three dressings were well tolerated (overall rate of normal skin >89 %, and no allergic reactions were observed for 99 % of all dressings). Skin status was not different between groups. Redness was the most common adverse event (4.9 %) (ESA 3.6, Table S7) followed by bleeding under the dressing (4.6 %). We identified 31 doubtful and nine violent reactions, all analysed and followed up until resolution; nevertheless, none of them could be attributed directly to the dressings. The doubtful reactions resolved spontaneously within 24 h; violent reactions, which affected five patients, were associated with an exacerbation under the dressing of a systemic reaction related to another event (medication allergy, comorbidity, etc.).
Discussion
The results show an accurate and comprehensive picture all intravascular post-insertion complications found in intensive care, highlighting an important overall complication incidence rate (60.9/1000 catheter-days) involving more than one-fourth of all IVCs.
Another important aspect pinpointed by the study was the relevance of deep-vein thrombosis (DVT) as the most frequent very severe complication, exceeding infections in number and in severity. Actually, catheter-related infections incidence, consistent with other studies [15–18], remained low (0.3/1000 catheter-days) and occurred only in three cases. In contrast, we identified 33 cases of DVT that, as found in precedent studies in intensive care settings [9, 19–21], had a high impact on the patient’s outcome; most of them required long-term medical treatment (anticoagulant) or urgent surgical intervention. Catheterization is an important risk factor for developing DVT. DVT is also related to insertion site infection, as already suggested by other studies [21–23], but also catheter type and size [24] may facilitate the occurrence of thrombotic events. In our study, HDCs represent an important share of all severe and very severe complications, in particular thrombosis. Increased severity and complication frequency with these devices may be related to greater patient fragility, as this particular group of patients has an already higher gravity score at ICU admission (ESA 3.3, Table S4), or to a higher risk of introduction of organisms during dialysis procedures [25, 26].
Peripheral venous catheters were the most frequently inserted devices. As in recent studies [27, 28], PVCs were also associated with the highest number of complications, revealing the weakness of this access in intensive settings. Complications were often related to dysfunction (15.9/1000 catheter-days), but the most serious complications were moderate (7.1/1000 catheter-days) and severe phlebitis and extravasations (1.1/1000 catheter-days), similar in gravity to those found in the literature [27, 29]. On the other hand, CVCs had a low complication incidence rate (10.9/1000 catheter-days), but some of the events, in particular DVT and infection, remained very severe. Disparities in density rates among PVCs and CVCs raise the question of the adequacy of vascular access in intensive care, and the interest of carefully balancing risks and benefits as proposed recently by Bouza and Fernandez-Ruiz [30, 31].
Improvements in catheter and dressing technologies and compliance with catheter maintenance bundles have provided real efficiency in catheter-related infection prevention [32–35]. The impact of dressings has been demonstrated in different studies, in PVCs [11, 36], ACs [37] and CVCs [38, 39], and the results are similar to those obtained in our study. However, the present study did not find any statistically significant difference between the different dressing groups with respect to complication occurrence, dressing disruption rates or skin tolerance.
Our study has some major limitations, in particular the single centre and open-label design. For obvious reasons of dressing differences, study groups were not blinded for nurses and physicians; but complication reports were anonymous and their analysis was performed without displaying the randomization group. Ultrasound exploration and peripheral venous catheter tips analysis were performed only on request; therefore, silent deep venous thrombosis or colonization of peripheral catheters could have remained unnoticed. Unfortunately, we did not collect initially ventilator-free days data, an end point that would have better described ICU resource utilization. Finally, further validation of the Delphi process would have ensured higher objectivity, as some items (like catheter-tip colonization) remain subject to discussion.
Conclusion
Maintaining reliable and adapted vascular access is a complex process, involving numerous clinical factors, staff competencies and a careful balance between risks and benefits when choosing the kind of access and the insertion site. None of the new dressings analysed in this study decreased complication or dressing disruption rates. The main contribution of this study was to put into perspective the various complications related to vascular access in intensive care.
Despite important progress on serious adverse events such as catheter-related infections, other complications remain a challenge, in particular DVT, and additional efforts are needed to reduce iatrogenic complications related to intravascular access in intensive care settings.
References
Savey A, Machut A (eds) (2012) Institut national de veille sanitaire (INVS) Surveillance des infections nosocomiales en réanimation adulte. Résultats REA-Raisin, France, résultats 2011. INVS, Lyon
Renaud B, Brun-Buisson C (2001) Outcomes of primary and catheter-related bacteremia. A cohort and case-control study in critically ill patients. Am J Respir Crit Care Med 163:1584–1590
Pittet D, Tarara D, Wenzel RP (1994) Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA 271:1598–1601
Mermel LA (2011) What is the predominant source of intravascular catheter infections? Clin Infect Dis 52:211–212
Timsit JF, L’Heriteau F, Lepape A, Francais A, Ruckly S, Venier AG, Jarno P, Boussat S, Coignard B, Savey A (2012) A multicentre analysis of catheter-related infection based on a hierarchical model. Intensive Care Med 38:1662–1672
Mermel LA (2007) Prevention of central venous catheter-related infections: what works other than impregnated or coated catheters? J Hosp Infect 65(Suppl 2):30–33
Maki DG, Kluger DM, Crnich CJ (2006) The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc 81:1159–1171
Timsit JF, Bouadma L, Ruckly S, Schwebel C, Garrouste-Orgeas M, Bronchard R, Calvino-Gunther S, Laupland K, Adrie C, Thuong M, Herault MC, Pease S, Arrault X, Lucet JC (2012) Dressing disruption is a major risk factor for catheter-related infections. Crit Care Med 40:1707–1714
Timsit JF, Farkas JC, Boyer JM, Martin JB, Misset B, Renaud B, Carlet J (1998) Central vein catheter-related thrombosis in intensive care patients: incidence, risks factors, and relationship with catheter-related sepsis. Chest 114:207–213
Gabriel J (2010) Vascular access devices: securement and dressings. Nurs Stand 24:41–46
Bausone-Gazda D, Lefaiver CA, Walters SA (2010) A randomized controlled trial to compare the complications of 2 peripheral intravenous catheter-stabilization systems. J Infus Nurs 33:371–384
Rutledge LF, DeCabooter DP, Walters SA, Bernatchez SF (2015) Catheter securement systems: comparison of two investigational devices to a sutureless securement device, a securement dressing, and sutures in a pig model. Intensive Care Med Exp 3:60
Ullman AJ, Cooke ML, Mitchell M, Lin F, New K, Long DA, Mihala G, Rickard CM (2016) Dressing and securement for central venous access devices (CVADs): a Cochrane systematic review. Int J Nurs Stud 59:177–196
Marsh N, Webster J, Mihala G, Rickard CM (2015) Devices and dressings to secure peripheral venous catheters to prevent complications. Cochrane Database Syst Rev CD011070
Timsit JF, Mimoz O, Mourvillier B, Souweine B, Garrouste-Orgeas M, Alfandari S, Plantefeve G, Bronchard R, Troche G, Gauzit R, Antona M, Canet E, Bohe J, Lepape A, Vesin A, Arrault X, Schwebel C, Adrie C, Zahar JR, Ruckly S, Tournegros C, Lucet JC (2012) Randomized controlled trial of chlorhexidine dressing and highly adhesive dressing for preventing catheter-related infections in critically ill adults. Am J Respir Crit Care Med 186:1272–1278
Timsit JF, Schwebel C, Bouadma L, Geffroy A, Garrouste-Orgeas M, Pease S, Herault MC, Haouache H, Calvino-Gunther S, Gestin B, Armand-Lefevre L, Leflon V, Chaplain C, Benali A, Francais A, Adrie C, Zahar JR, Thuong M, Arrault X, Croize J, Lucet JC (2009) Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: a randomized controlled trial. JAMA 301:1231–1241
Lucet JC, Boudama L, Zahar JR, Schwebel C, Geffory A, Pease S, Herault MC, Haouache H, Adrie C, Thuong M, Francais A, Garrouste-Orgeas M, Timsit JF (2010) Infectious risk associated with arterial catheters compared to central venous catheters. Crit Care Med 38:552–559
Mimoz O, Lucet JC, Kerforne T, Pascal J, Souweine B, Goudet V, Mercat A, Bouadma L, Lasocki S, Alfandari S, Friggeri A, Wallet F, Allou N, Ruckly S, Balayn D, Lepape A, Timsit JF (2015) Skin antisepsis with chlorhexidine-alcohol versus povidone iodine-alcohol, with and without skin scrubbing, for prevention of intravascular-catheter-related infection (CLEAN): an open-label, multicentre, randomised, controlled, two-by-two factorial trial. Lancet 386:2069–2077
Minet C, Potton L, Bonadona A, Hamidfar-Roy R, Somohano CA, Lugosi M, Cartier JC, Ferretti G, Schwebel C, Timsit JF (2015) Venous thromboembolism in the ICU: main characteristics, diagnosis and thromboprophylaxis. Crit Care 19:287
Baskin JL, Pui CH, Reiss U, Wilimas JA, Metzger ML, Ribeiro RC, Howard SC (2009) Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet 374:159–169
Malinoski D, Ewing T, Bhakta A, Schutz R, Imayanagita B, Casas T, Woo N, Margulies D, Barrios C, Lekawa M, Chung R, Bukur M, Kong A (2013) Which central venous catheters have the highest rate of catheter-associated deep venous thrombosis: a prospective analysis of 2,128 catheter days in the surgical intensive care unit. J Trauma Acute Care Surg 74:454–460
Leung A, Heal C, Perera M, Pretorius C (2015) A systematic review of patient-related risk factors for catheter-related thrombosis. J Thromb Thrombolysis 40:363–373
Ge X, Cavallazzi R, Li C, Pan SM, Wang YW, Wang FL (2012) Central venous access sites for the prevention of venous thrombosis, stenosis and infection. Cochrane Database Syst Rev 3:CD004084
Moretti EW, Ofstead CL, Kristy RM, Wetzler HP (2005) Impact of central venous catheter type and methods on catheter-related colonization and bacteraemia. J Hosp Infect 61:139–145
Betjes MG (2011) Prevention of catheter-related bloodstream infection in patients on hemodialysis. Nat Rev Nephrol 7:257–265
Wilson P, Lertdumrongluk P, Leray-Moragues H, Chenine-Koualef L, Patrier L, Canaud B (2012) Prevention and management of dialysis catheter complications in the intensive care unit. Blood Purif 34:194–199
Cicolini G, Bonghi AP, Di Labio L, Di Mascio R (2009) Position of peripheral venous cannulae and the incidence of thrombophlebitis: an observational study. J Adv Nurs 65:1268–1273
Gonzalez Lopez JL, Arribi Vilela A, Fernandez del Palacio E, Olivares Corral J, Benedicto Marti C, Herrera Portal P (2014) Indwell times, complications and costs of open vs closed safety peripheral intravenous catheters: a randomized study. J Hosp Infect 86:117–126
Ricard JD, Salomon L, Boyer A, Thiery G, Meybeck A, Roy C, Pasquet B, Le Miere E, Dreyfuss D (2013) Central or peripheral catheters for initial venous access of ICU patients: a randomized controlled trial. Crit Care Med 41:2108–2115
Bouza E, Guembe M, Munoz P (2010) Selection of the vascular catheter: can it minimise the risk of infection? Int J Antimicrob Agents 36(Suppl 2):S22–S25
Fernandez-Ruiz M, Carretero A, Diaz D, Fuentes C, Gonzalez JI, Garcia-Reyne A, Aguado JM, Lopez-Medrano F (2014) Hospital-wide survey of the adequacy in the number of vascular catheters and catheter lumens. J Hosp Med 9:35–41
Vandijck DM, Labeau SO, Secanell M, Rello J, Blot SI (2009) The role of nurses working in emergency and critical care environments in the prevention of intravascular catheter-related bloodstream infections. Int Emerg Nurs 17:60–68
Shapey IM, Foster MA, Whitehouse T, Jumaa P, Bion JF (2009) Central venous catheter-related bloodstream infections: improving post-insertion catheter care. J Hosp Infect 71:117–122
Eggimann P, Harbarth S, Constantin MN, Touveneau S, Chevrolet JC, Pittet D (2000) Impact of a prevention strategy targeted at vascular-access care on incidence of infections acquired in intensive care. Lancet 355:1864–1868
Guerin K, Wagner J, Rains K, Bessesen M (2010) Reduction in central line-associated bloodstream infections by implementation of a postinsertion care bundle. Am J Infect Control 38:430–433
Schears GJ (2006) Summary of product trials for 10,164 patients: comparing an intravenous stabilizing device to tape. J Infus Nurs 29:225–231
Reynolds H, Taraporella K, Tower M, Mihala G, Tuffaha HW, Fraser JF, Rickard CM (2015) Novel technologies can provide effective dressing and securement for peripheral arterial catheters: a pilot randomised controlled trial in the operating theatre and the intensive care unit. Aust Crit Care 28:140–148
Webster J, Gillies D, O’Riordan E, Sherriff KL, Rickard CM (2011) Gauze and tape and transparent polyurethane dressings for central venous catheters. Cochrane Database Syst Rev CD003827
Vokurka S, Bystricka E, Visokaiova M, Scudlova J (2009) Once- versus twice-weekly changing of central venous catheter occlusive dressing in intensive chemotherapy patients: results of a randomized multicenter study. Med Sci Monit 15:CR107–CR110
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
This study was financed by a research grant from 3M™ Health Care (Neuss, Germany). The sponsors of the study had no role in study design, data collection, analysis and interpretation, or writing of the report. Sponsors had no role either in the proposal or the election of the Delphi members. Corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. The corresponding author states that there is no further conflict of interest to disclose.
Additional information
Take-home message:
Post-insertion intravascular access complications are frequent and underestimated iatrogenic events affecting one fourth of all intravascular devices in intensive care settings. This randomized controlled trial reported a high, mainly non-infectious, life-threatening rate of complications not prevented by new adhesive dressings.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Günther, S.C., Schwebel, C., Hamidfar-Roy, R. et al. Complications of intravascular catheters in ICU: definitions, incidence and severity. A randomized controlled trial comparing usual transparent dressings versus new-generation dressings (the ADVANCED study). Intensive Care Med 42, 1753–1765 (2016). https://doi.org/10.1007/s00134-016-4582-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-016-4582-2