Abstract
Purpose
The interpretation of septic shock trial data is profoundly affected by patients, control intervention, co-interventions and selected outcome measures. We evaluated the reporting of control groups in recent septic shock trials.
Methods
We searched for original articles presenting randomized clinical trials (RCTs) in adult septic shock patients from 2006 to 2016. We included RCTs focusing on septic shock patients with at least two parallel groups and at least 50 patients in the control group. We selected and evaluated data items regarding patients, control group characteristics, and mortality outcomes, and calculated a data completeness score to provide an overall view of quality of reporting.
Results
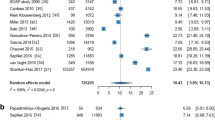
A total of 24 RCTs were included (mean n = 287 patients and 71 % of eligible patients were randomized). Of the 24 studies, 14 (58 %) presented baseline data on vasopressors and 58 % the proportion of patients with elevated lactate values. Five studies (21 %) provided data to estimate the proportion of septic shock patients fulfilling the Sepsis-3 definition. The mean data completeness score was 19 out of 36 (range 8–32). Of 18 predefined control group characteristics, a mean of 8 (range 2–17) were reported. Only 2 (8 %) trials provided adequate data to confirm that their control group treatment represented usual care.
Conclusions
Recent trials in septic shock provide inadequate data on the control group treatment and hemodynamic values. We propose a standardized trial dataset to be created and validated, comprising characteristics of patient population, interventions administered, hemodynamic values achieved, surrogate organ dysfunction, and mortality outcomes, to allow better analysis and interpretation of future trial results.

Similar content being viewed by others
References
Shankar-Hari M, Phillips GS, Levy ML et al (2016) Developing a new definition and assessing new clinical criteria for septic shock: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315:775–787
Deans KJ, Minneci PC, Suffredini AF et al (2007) Randomization in clinical trials of titrated therapies: unintended consequences of using fixed treatment protocols. Crit Care Med 35:1509–1516
Takala J (2009) Better conduct of clinical trials: the control group in critical care trials. Crit Care Med 37(1 Suppl):S80–S90
Investigators PROCESS, Yealy DM, Kellum JA et al (2014) A randomized trial of protocol-based care for early septic shock. N Engl J Med 370:1683–1693
ARISE Investigators, ANZICS Clinical Trials Group, Peake S et al (2014) Goal-directed resuscitation for patients with early septic shock. N Engl J Med 371:1496–1506
Mouncey PR, Osborn T, Power GS, Trial Investigators PROMISE et al (2015) Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 372:1301–1311
Caironi P, Tognoni G, Masson S, Albios Study Investigators et al (2014) Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med 370:1412–1421
De Backer D, Biston P, Devriendt J et al (2010) Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 362:779–789
Brunkhorst FM, Oppert M, Marx G et al (2012) Effect of empirical treatment with moxifloxacin and meropenem vs. meropenem on sepsis-related organ dysfunction in patients with severe sepsis: a randomized trial. JAMA 307:2390–2399
Vincent JL, Marshall JC, Dellinger RP et al (2015) Talactoferrin in severe sepsis: results from the phase II/III Oral tAlactoferrin in Severe sepsIS Trial. Crit Care Med 43:1832–1838
Lin SM, Huang CD, Lin HC, Liu CY, Wang CH, Kuo HP (2006) A modified goal-directed protocol improves clinical outcomes in intensive care unit patients with septic shock: a randomized controlled trial. Shock 26:551–557
Annane D, Vignon P, Renault A et al (2007) Norepinephrine plus dobutamine versus epinephrine alone for management of septic shock: a randomised trial. Lancet 370:676–684
Werdan K, Pilz G, Bujdoso O et al (2007) Score-based immunoglobulin G therapy of patients with sepsis: the SBITS study. Crit Care Med 35:2693–2701
Sprung CL, Annane D, Keh D et al (2008) Hydrocortisone therapy for patients with septic shock. N Engl J Med 358:111–124
Stephens DP, Thomas JH, Higgins A et al (2008) Randomized, double-blind, placebo-controlled trial of granulocyte colony-stimulating factor in patients with septic shock. Crit Care Med 36:448–454
Russell JA, Walley KR, Singer J et al (2008) Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 358:877–887
Dhainaut JF, Antonelli M, Wright P et al (2009) Extended drotrecogin alfa (activated) treatment in patients with prolonged septic shock. Intensive Care Med 35:1187–1195
Palizas F, Dubin A, Regueira T et al (2009) Gastric tonometry versus cardiac index as resuscitation goals in septic shock: a multicenter, randomized, controlled trial. Crit Care 13:R44
Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA (2010) Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA 303:739–746
Patel GP, Grahe JS, Sperry M et al (2010) Efficacy and safety of dopamine versus norepinephrine in the management of septic shock. Shock 33:375–380
Annane D, Cariou A, Maxime V et al (2010) Corticosteroid treatment and intensive insulin therapy for septic shock in adults: a randomized controlled trial. JAMA 303:341–348
Huh JW, Choi HS, Lim CM et al (2011) Low-dose hydrocortisone treatment for patients with septic shock: a pilot study comparing 3 days with 7 days. Respirology 16:1088–1095
Ranieri VM, Thompson BT, Barie BS, PROWESS-SHOCK study group et al (2012) Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med 366:2055–2064
Perner A, Haase N, Guttormsen AB, Scandinavian Critical Care Trials Group (2012) Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med 367:124–134
Schortgen F, Clabault K, Katsahian S et al (2012) Fever control using external cooling in septic shock: a randomized controlled trial. Am J Respir Crit Care Med 185:1088–1095
Annane D, Timsit JF, Megarbane B et al (2013) Recombinant human activated protein C for adults with septic shock: a randomized controlled trial. Am J Respir Crit Care Med 187:1091–1097
Joannes-Boyau O, Honore PM, Perez P et al (2013) High-volume versus standard-volume haemofiltration for septic shock patients with acute kidney injury (IVOIRE study): a multicentre randomized controlled trial. Intensive Care Med 39:1535–1546
Holst LB, Haase N, Wetterslev J, TRISS trial group, Scandinavian Critical Care Trials Group et al (2014) Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med 371:1381–1391
Asfar P, Meziani F, Hamel JF, Investigators Sepsispam et al (2014) High versus low blood-pressure target in patients with septic shock. N Engl J Med 370:1583–1593
Lu NF, Zheng RQ, Lin H, Shao J, Yu JQ, Yang DG (2015) Improved sepsis bundles in the treatment of septic shock: a prospective clinical study. Am J Emerg Med 33:1045–1049
Payen DM, Guilhot J, Launey Y et al (2015) Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: a multicenter randomized control trial. Intensive Care Med 41:975–984
Singer M, Deutschman CS, Seymour CW et al (2016) The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315:801–810
Kavanagh BP, Nurok M (2015) Standardized intensive care—protocol misalignment and impact misattribution. Am J Respir Crit Care Med 193:17–22
Porter ME, Larsson S, Lee TH (2016) Standardizing patient outcomes measurement. N Engl J Med 374:504–506
Myles PS, Grocott MP, Boney O, Moonesinghe SR, COMPAC-StEP Group (2016) Standardizing end points in perioperative trials: towards a core and extended outcome set. Br J Anaesth 116:586–589
Schulz KF, Altman DG, Moher D, For the CONSORT group (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. J Clin Epidem 63:834–840
Annane D (2009) Improving clinical trials in the critically ill: unique challenge—sepsis. Crit Care Med 37:S117–S128
Acknowledgments
We would like to thank Information Specialist Tiina M. Heino, MSc from Terkko Library (Helsinki, Finland) for performing the literature searches, and medical editor Jeannie Wurz (Bern, Switzerland) for careful editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest regarding this contribution. AP was the principal investigator of 6S and TRISS trials, PH co-authored the TRISS trial, and VP co-authored TRISS and ARISE trials and contributed as an investigator to EXTENDED APC, PROWESS-SHOCK, and 6S trials.
Additional information
Take-home message: Recent trials in septic shock provide inadequate data on the treatment given the control group and the hemodynamic values achieved. We propose a standardized trial dataset comprising characteristics of patient population, co-interventions administered, hemodynamic values achieved, surrogate organ dysfunction, and mortality outcomes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pettilä, V., Hjortrup, P.B., Jakob, S.M. et al. Control groups in recent septic shock trials: a systematic review. Intensive Care Med 42, 1912–1921 (2016). https://doi.org/10.1007/s00134-016-4444-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-016-4444-y