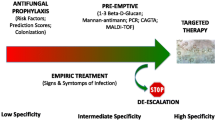
Abstract
Purpose
Systemic antifungal therapy (SAT) of invasive candidiasis needs to be initiated immediately upon clinical suspicion. Controversies exist about adequate time and potential harm of antifungal de-escalation (DE) in documented and suspected candidiasis in ICU patients. Our objective was to investigate whether de-escalation within 5 days of antifungal initiation is associated with an increase of the 28-day mortality in SAT-treated non-neutropenic adult ICU patients.
Methods
From the 835 non-neutropenic adults recruited in the multicenter prospective observational AmarCAND2 study, we selected the patients receiving systemic antifungal therapy for a documented or suspected invasive candidiasis in the ICU and who were still alive 5 days after SAT initiation. They were included into two groups according to the occurrence of observed SAT de-escalation before day 6. The average causal SAT de-escalation effect on 28-day mortality was evaluated by using a double robust estimation.
Results
Among the 647 included patients, early de-escalation at day 5 after antifungal initiation occurred in 142 patients (22 %), including 48 (34 %) patients whose SAT was stopped before day 6. After adjustment for the baseline confounders, early SAT de-escalation was the solely factor not associated with increased 28-day mortality (RR 1.12, 95 % CI 0.76–1.66).
Conclusion
In non-neutropenic critically ill adult patients with documented or suspected invasive candidiasis, SAT de-escalation within 5 days was not related to increased day-28 mortality but it was associated with decreased SAT consumption. These results suggest for the first time that SAT de-escalation may be safe in these patients.


Similar content being viewed by others
References
Rangel-Frausto MS, Wiblin T, Blumberg HM, Saiman L, Patterson J, Rinaldi M, Pfaller M, Edwards JE, Jarvis W, Dawson J, Wenzel RP (1999) National epidemiology of mycoses survey (NEMIS): variations in rates of bloodstream infections due to Candida species in seven surgical intensive care units and six neonatal intensive care units. Clin Infect Dis 29:253–258
Tabah A, Koulenti D, Laupland K, Misset B, Valles J, Bruzzi de Carvalho F, Paiva JA, Cakar N, Ma X, Eggimann P, Antonelli M, Bonten MJM, Csomos A, Krueger WA, Mikstacki A, Lipman J, Depuydt P, Vesin A, Garrouste-Orgeas M, Zahar J-R, Blot S, Carlet J, Brun-Buisson C, Martin C, Rello J, Dimopoulos G, Timsit J-F (2012) Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: the EUROBACT International Cohort Study. Intensive Care Med 38:1930–1945
Blumberg HM, Jarvis WR, Soucie JM, Edwards JE, Patterson JE, Pfaller MA, Rangel-Frausto MS, Rinaldi MG, Saiman L, Wiblin RT, Wenzel RP (2001) Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS Prospective Multicenter Study. Clin Infect Dis 33:177–186
Bassetti M, Righi E, Ansaldi F, Merelli M, Trucchi C, Cecilia T, De Pascale G, Diaz-Martin A, Luzzati R, Rosin C, Lagunes L, Trecarichi EM, Sanguinetti M, Posteraro B, Garnacho-Montero J, Sartor A, Rello J, Rocca GD, Antonelli M, Tumbarello M (2014) A multicenter study of septic shock due to candidemia: outcomes and predictors of mortality. Intensive Care Med 40:839–845
Garrouste-Orgeas M, Timsit JF, Tafflet M, Misset B, Zahar J-R, Soufir L, Lazard T, Jamali S, Mourvillier B, Cohen Y, De Lassence A, Azoulay E, Cheval C, Descorps-Declere A, Adrie C, Costa de Beauregard M-A, Carlet J, OUTCOMEREA Study Group (2006) Excess risk of death from intensive care unit-acquired nosocomial bloodstream infections: a reappraisal. Clin Infect Dis 42:1118–1126
Kollef M, Micek S, Hampton N, Doherty JA, Kumar A (2012) Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis 54:1739–1746
Pittet D, Li N, Woolson RF, Wenzel RP (1997) Microbiological factors influencing the outcome of nosocomial bloodstream infections: a 6-year validated, population-based model. Clin Infect Dis 24:1068–1078
Wisplinghoff H, Seifert H, Tallent SM, Bischoff T, Wenzel RP, Edmond MB (2003) Nosocomial bloodstream infections in pediatric patients in United States hospitals: epidemiology, clinical features and susceptibilities. Pediatr Infect Dis J 22:686–691
Grim SA, Berger K, Teng C, Gupta S, Layden JE, Janda WM, Clark NM (2012) Timing of susceptibility-based antifungal drug administration in patients with Candida bloodstream infection: correlation with outcomes. J Antimicrob Chemother 67:707–714
Köck R, Eißing LC, Boschin MG, Ellger B, Horn D, Idelevich EA, Becker K (2013) Evaluation of bactec mycosis IC/F and Plus Aerobic/F blood culture bottles for detection of Candida in the presence of antifungal agents. J Clin Microbiol 51:3683–3687
Eggimann P, Pittet D (2014) Candida colonization index and subsequent infection in critically ill surgical patients: 20 years later. Intensive Care Med 40:1429–1448
Azoulay E, Dupont H, Tabah A, Lortholary O, Stahl J-P, Francais A, Martin C, Guidet B, Timsit J-F (2012) Systemic antifungal therapy in critically ill patients without invasive fungal infection. Crit Care Med 40:813–822
Alexander BD, Johnson MD, Pfeiffer CD, Jiménez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA (2013) Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 56:1724–1732
Saraya T, Tanabe K, Araki K, Yonetani S, Makino H, Watanabe T, Tsujimoto N, Takata S, Kurai D, Ishii H, Miyazaki Y, Takizawa H, Goto H (2014) Breakthrough invasive Candida glabrata in patients on micafungin: a novel FKS gene conversion correlated with sequential elevation of MIC. J Clin Microbiol 52:2709–2712
Ostrosky-Zeichner L, Shoham S, Vazquez J, Reboli A, Betts R, Barron MA, Schuster M, Judson MA, Revankar SG, Caeiro JP, Mangino JE, Mushatt D, Bedimo R, Freifeld A, Nguyen MH, Kauffman CA, Dismukes WE, Westfall AO, Deerman JB, Wood C, Sobel JD, Pappas PG (2014) MSG-01: a randomized, double-blind, placebo-controlled trial of caspofungin prophylaxis followed by preemptive therapy for invasive candidiasis in high-risk adults in the critical care setting. Clin Infect Dis 58:1219–1226
León C, Ruiz-Santana S, Saavedra P, Galván B, Blanco A, Castro C, Balasini C, Utande-Vázquez A, González de Molina FJ, Blasco-Navalproto MA, López MJ, Charles PE, Martín E, Hernández-Viera MA, Cava Study Group (2009) Usefulness of the “Candida score” for discriminating between Candida colonization and invasive candidiasis in non-neutropenic critically ill patients: a prospective multicenter study. Crit Care Med 37:1624–1633
Ostrosky-Zeichner L, Sable C, Sobel J, Alexander BD, Donowitz G, Kan V, Kauffman CA, Kett D, Larsen RA, Morrison V, Nucci M, Pappas PG, Bradley ME, Major S, Zimmer L, Wallace D, Dismukes WE, Rex JH (2007) Multicenter retrospective development and validation of a clinical prediction rule for nosocomial invasive candidiasis in the intensive care setting. Eur J Clin Microbiol Infect Dis 26:271–276
Bailly S, Bouadma L, Azoulay E, Orgeas MG, Adrie C, Souweine B, Schwebel C, Maubon D, Hamidfar-Roy R, Darmon M, Wolff M, Cornet M, Timsit J-F (2015) Failure of empirical systemic antifungal therapy in mechanically ventilated critically ill patients. Am J Respir Crit Care Med 191:1139–1146
Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, Meersseman W, Akova M, Arendrup MC, Arikan-Akdagli S, Bille J, Castagnola E, Cuenca-Estrella M, Donnelly JP, Groll AH, Herbrecht R, Hope WW, Jensen HE, Lass-Flörl C, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Viscoli C, Ullmann AJ, ESCMID Fungal Infection Study Group (2012) ESCMID guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 18(Suppl 7):19–37
Pappas PG, Kauffman CA, Andes D, Benjamin DK, Calandra TF, Edwards JE, Filler SG, Fisher JF, Kullberg B-J, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD, Infectious Diseases Society of Americia (2009) Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 48:503–535
Vazquez J, Reboli AC, Pappas PG, Patterson TF, Reinhardt J, Chin-Hong P, Tobin E, Kett DH, Biswas P, Swanson R (2014) Evaluation of an early step-down strategy from intravenous anidulafungin to oral azole therapy for the treatment of candidemia and other forms of invasive candidiasis: results from an open-label trial. BMC Infect Dis 14:97
Takesue Y, Ueda T, Mikamo H, Oda S, Takakura S, Kitagawa Y, Kohno S, ACTIONs Project (2015) Management bundles for candidaemia: the impact of compliance on clinical outcomes. J Antimicrob Chemother 70:587–593
Bang H, Robins JM (2005) Doubly robust estimation in missing data and causal inference models. Biometrics 61:962–973
Bailly S, Pirracchio R, Timsit JF (2015) What’s new in the quantification of causal effects from longitudinal cohort studies: a brief introduction to marginal structural models for intensivists. Intensive Care Med. doi:10.1007/s00134-015-3919-6
Zhang M, Schaubel DE (2012) Contrasting treatment-specific survival using double-robust estimators. Stat Med 31:4255–4268
Funk MJ, Westreich D, Wiesen C, Stürmer T, Brookhart MA, Davidian M (2011) Doubly robust estimation of causal effects. Am J Epidemiol 173:761–767
Lortholary O, Renaudat C, Sitbon K, Madec Y, Denoeud-Ndam L, Wolff M, Fontanet A, Bretagne S, Dromer F, French Mycosis Study Group (2014) Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002–2010). Intensive Care Med 40:1303–1312
Andes D, Forrest A, Lepak A, Nett J, Marchillo K, Lincoln L (2006) Impact of antimicrobial dosing regimen on evolution of drug resistance in vivo: fluconazole and Candida albicans. Antimicrob Agents Chemother 50:2374–2383
Fekkar A, Dannaoui E, Meyer I, Imbert S, Brossas JY, Uzunov M, Mellon G, Nguyen S, Guiller E, Caumes E, Leblond V, Mazier D, Fievet MH, Datry A (2014) Emergence of echinocandin-resistant Candida spp. in a hospital setting: a consequence of 10 years of increasing use of antifungal therapy? Eur J Clin Microbiol Infect Dis 33:1489–1496
Khan Z, Ahmad S, Joseph L, Al-Obaid K (2014) Isolation of cholesterol-dependent, multidrug-resistant Candida glabrata strains from blood cultures of a candidemia patient in Kuwait. BMC Infect Dis 14:188
Lewis JS, Wiederhold NP, Wickes BL, Patterson TF, Jorgensen JH (2013) Rapid emergence of echinocandin resistance in Candida glabrata resulting in clinical and microbiologic failure. Antimicrob Agents Chemother 57:4559–4561
Shields RK, Nguyen MH, Press EG, Updike CL, Clancy CJ (2013) Caspofungin MICs correlate with treatment outcomes among patients with Candida glabrata invasive candidiasis and prior echinocandin exposure. Antimicrob Agents Chemother 57:3528–3535
Bizerra FC, Jimenez-Ortigosa C, Souza ACR, Breda GL, Queiroz-Telles F, Perlin DS, Colombo AL (2014) Breakthrough candidemia due to multidrug-resistant Candida glabrata during prophylaxis with a low dose of micafungin. Antimicrob Agents Chemother 58:2438–2440
Bal AM, Shankland GS, Scott G, Imtiaz T, Macaulay R, McGill M (2014) Antifungal step-down therapy based on hospital intravenous to oral switch policy and susceptibility testing in adult patients with candidaemia: a single centre experience. Int J Clin Pract 68:20–27
Tissot F, Lamoth F, Hauser PM, Orasch C, Flückiger U, Siegemund M, Zimmerli S, Calandra T, Bille J, Eggimann P, Marchetti O (2013) Beta-glucan antigenemia anticipates diagnosis of blood culture-negative intraabdominal candidiasis. Am J Respir Crit Care Med 188:1100–1109
Poissy J, Sendid B, Damiens S, Ichi Ishibashi K, François N, Kauv M, Favory R, Mathieu D, Poulain D (2014) Presence of Candida cell wall derived polysaccharides in the sera of intensive care unit patients: relation with candidaemia and Candida colonisation. Crit Care 18:R135
Acknowledgments
Members of the independent scientific committee: Olivier Leroy (MD, medical intensivist), Jean-François Timsit (MD, PhD, medical intensivist, epidemiologist), Jean-Pierre Gangneux (MD, PhD, mycologist), Elie Azoulay (MD, PhD, medical intensivist), Jean-Michel Constantin (MD, PhD, anesthesiologist and intensivist), Hervé Dupont (MD, PhD, anesthesiologist and intensivist), Olivier Lortholary (MD, PhD, infectious diseases specialist), Jean-Paul Mira (MD, PhD, medical intensivist), Philippe Montravers (MD, PhD, anesthesiologist and intensivist), Pierre-François Perrigault (MD, anesthesiologist and intensivist). Manuscript preparation: the authors thank Celine Feger (MD; EMIBiotech), who provided assistance in preparing and editing the manuscript and Romain Pirrachio (MD, PhD; University of California, San Francisco) who reviewed statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by MSD France, which was the sponsor.
Conflicts of interest
Authors had full access to the database, and the statistical analyses were conducted by an academic team: Biostatistics Unit, Lille University Hospital, Lille, France, which worked in full independence from MSD.
EA has been a consultant to Astellas, Alexion, Cubist, Gilead, and MSD, and has benefited from grants to his research unit from Gilead and Pfizer. J-MC has been a consultant to MSD. HD has been a consultant to Astellas, Gilead, Cubist, Astrazeneca, Merck, and Pfizer. J-PG has been a consultant to Astellas, Gilead, Merck, and Pfizer. DG has benefited from grants of the Principality of Monaco to his research unit. O Leroy has been consultant to Astellas, Gilead, Merck, Novartis, Pfizer, and Sanofi. O Lortholary has been consultant to Gilead Sciences and Novartis and member of the speaker’s bureau of Astellas, Basilea, Merck, Pfizer, and Sanofi. J-PM has been a consultant to Astellas, Gilead, MSD, and LFB. PM has been a consultant to Astra-Zeneca, Cubist, MSD, Pfizer, and TMC. P-FP has been a consultant to MSD and Pfizer. JFT has given lectures for symposiums set up by Astellas, Pfizer, MSD, 3M, Novartis, and Gilead; has benefited from unrestricted research grants to his research unit from 3M, MSD, and Astellas; and has been a consultant involved in scientific boards for MSD, 3M, and Bayer. SB has no conflict of interest.
Additional information
Take-home message: Early de-escalation of systemic antifungal treatments is not consensual. A causal analysis based on a multicenter prospective cohort in 87 French ICUs has shown that antifungal de-escalation within a 5-day interval is safe in SAT-treated non-neutropenic adult intensive care unit patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bailly, S., Leroy, O., Montravers, P. et al. Antifungal de-escalation was not associated with adverse outcome in critically ill patients treated for invasive candidiasis: post hoc analyses of the AmarCAND2 study data. Intensive Care Med 41, 1931–1940 (2015). https://doi.org/10.1007/s00134-015-4053-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-015-4053-1