Abstract
Background
Severe injury triggers a complex systemic immune response which may result in significant respiratory compromise, including the development of acute respiratory distress syndrome (ARDS). No randomized clinical trial has assessed the role of nutritional interventions to limit respiratory complications.
Methods
This was a single-center, prospective, randomized, comparative, double-blind, controlled study of patients with severe trauma requiring mechanical ventilation. Patients were randomly assigned to receive either a control formula (n = 58) or a formula enriched with eicosapentaenoic acid (EPA), gamma-linolenic acid (GLA) and antioxidants (n = 62) at time of admission to the intensive care unit (ICU). Primary outcome measures included the level of oxygenation (PaO2/FiO2 ratio, PF ratio) on days 4 and 8, incidence of acute lung injury (ALI) and/or ARDS and length of ventilation. The development of infectious complications and fatty acid red blood cell membrane composition were also assessed.
Results
In this intention-to-treat population, no significant differences between the control and study groups were found for the PF ratio at day 4 (213.7 ± 85.6 vs. 227.2 ± 67.7, respectively; P = 0.24) and day 8 (187.8 ± 65.2 vs. 188.9 ± 56.0, respectively; P = 0.82), the incidence of ARDS/ALI (24.1 vs. 29.0 %, respectively; P = 0.68), length of ventilation time (13.6 ± 10.7 vs. 17.0 ± 15.1 days, respectively; P = 0.15), duration of ICU stay (16.4 ± 11.3 vs. 19.5 ± 15.3 days, respectively; P = 0.21) and 28-day mortality (8.6 vs. 12.9 %, respectively P = 0.56). While the study group showed a significant increase in EPA and GLA concentrations at day 4 (P = 0.05) and day 8 (P < 0.001), the Omega-3 Index (O-3I) failed to reach those suggested as being optimal to obtain clinical efficacy. The significantly higher incidence of bacteremia noted in the study group (P = 0.03) was associated with a higher number of patients with multiple trauma and a higher red blood cell transfusion requirement (P = 0.008).
Conclusion
This study failed to show a significant benefit for the preemptive use of the study formula in patients with severe trauma. Additional studies need to be performed in which the amount of supplementation is targeted to a potentially measurable endpoint, e.g. the O-3I.
Similar content being viewed by others
Introduction
The initial stress response to severe trauma is characterized by an inflammatory state mediated by the innate immune system. Patients who survive this stage may subsequently develop a compensatory anti-inflammatory response syndrome characterized by suppression of cell-mediated immunity and a predisposition to severe sepsis, nosocomial infections, as well as the acute respiratory distress syndrome (ARDS), multiple organ dysfunction and ultimately even death [1].
Nutrient administration to the critically ill not only supplies energy substrates and protein to support metabolic needs, but may also have a role in attenuating the injurious components of inflammation while maintaining adaptive immunity. In fact, the early initiation of enteral nutrition (EN) has been shown to improve outcomes in severely injured trauma patients [2], and the administration of arginine, fish oil and nucleotides has been recommended for patients with moderate trauma to target immune modulation [3, 4]. In addition, the use of continuous nutritional support enriched with eicosapentaenoic acid (EPA), gamma-linolenic acid (GLA) and antioxidants has been shown to improve oxygenation, shorten the length of ventilation and length of stay in the intensive care unit (ICU) and decrease the inflammatory process in patients suffering from ARDS or acute lung injury (ALI) and sepsis [5–7]. Such results have led to strong recommendations for the use of these nutrients [4, 8], but the findings have not been universally consistent, and others studies, using mainly EPA as a bolus, failed to show a beneficial effect [9–11].
Many severely injured patients have well-described risk factors for the ARDS, including multiple trauma with a high Injury Severity Score (ISS) and thoracic injury or pulmonary contusions [12]. Increased plasma levels of pro-inflammatory mediators, including interleukin-8 (IL-8), IL-6, IL-1b, IL-10, IL-1ra and tumor necrosis factor alpha, have been demonstrated in bronchoalveolar lavage from such patients, supporting a central role of immunologically mediated mechanisms [13].
In view of these findings, approaches to modulate the early posttraumatic inflammatory responses to prevent additional secondary lung damage in multiple trauma patients have become relevant [14]. A study that includes only multiple trauma patients with the aim of limiting respiratory complications through the utilization of nutritional support with EPA and GLA and antioxidants has to our knowledge not been performed. The primary purpose of our study was, therefore, to assess the effects of an EN formula enriched with EPA, GLA and antioxidants, started upon admission of the patient to the ICU, on respiratory parameters in patients with multiple trauma requiring mechanical ventilation. The effects of this formulation on the respiratory parameters were compared to those of a control formula identical in terms of macronutrients. We also assessed the effect of the study formula on the development of infectious complications and on red blood cell membrane composition.
Methods
Patients
This single-center, prospective, randomized, comparative, double blind, controlled study was conducted in the general ICU of the Rabin Medical Center, Petah Tikva, Israel, a tertiary care, level 1 trauma center of a university-affiliated hospital, over a period of 44 months (from November 2010 to June 2014). The study protocol was approved by the local institutional review board, and informed consent was obtained prior to randomization either from the patient or his/her legal representative if possible, or from an independent physician where this was not possible. All patients between the ages of 18–90 years with a diagnosis of multiple trauma, defined as physical insults or injuries occurring simultaneously in more than one part of the body, or of isolated head trauma who required mechanical ventilation and had an anticipated ICU stay of ≥2 days were included in the study. Exclusion criteria were: (1) the presence of any contraindication for commencing EN within the first 36 h of ICU admission, such as mechanical or functional small bowel obstruction, high-output fistula, gastrointestinal tract discontinuity and/or surgeon reluctance to commence EN immediately following abdominal surgery; (2) treatment with immunosuppressive drugs; (3) second-/third-degree burns covering >66 % of body surface area; (4) pregnancy The study was registered at Clinical Trials as NCT01099501.
Randomization, intervention and data collection
Eligible patients were randomized into a control group who received a high-fat, low-carbohydrate enteral formula (Pulmocare; Ross laboratories, Chicago, IL) and a study group who received a formula enriched with supplemental EPA, GLA (Oxepa; Ross Laboratories) and antioxidants. The two feeds were decanted from their commercial packaging and presented at the bedside in a blinded manner. All healthcare workers involved in the daily care of the patients were blinded to the type of EN administered. Patients suffering from isolated head trauma were included sequentially in the overall randomization process (i.e. as a patient with multiple trauma or isolated head trauma). The two formulae were similar in terms of caloric content and protein and macronutrient composition (see "Appendix" Table 4). Randomization was achieved using a computer-based block randomization generated by a statistical software program which was concealed to all investigators apart from the principal investigator (PS).
EN, based on randomization, was delivered within 48 h of admission via a nasogastric or orogastric tube whose position was confirmed by X-ray. The amount of EN prescribed daily was meant to provide at least 80 % of all energy requirements as determined by measurement of resting energy expenditure (REE) (Deltatrac II; Datex-Ohmeda, GE Healthcare Finland Oy, Helsinki, Finland). REE measurements were performed before study inclusion (in the first 48 h after admission) and at intervals of 48–72 h thereafter. REE was calculated using Weir’s formula [15]. No preceding starvation period was required. If the fraction of inspired oxygen (FIO2) was ≥0.6, the Fagon formula was used [16]. Tolerance to EN was assessed by measuring gastric residual volume (GRV) every 8 h. In the presence of a GRV of >500 mL and/or vomiting or diarrhea >3 times/day, EN was stopped for 24 h and parenteral nutrition (PN) administered. If the GRV was between 150 and 500 mL, the EN rate was reduced to 50 % and/or prokinetic therapy (metoclopramide 10 mg × 3/day or erythromycin 150 mg × 3/day) initiated. If the GRV remained <500 mL and >80 % of caloric needs were met by EN alone, the feeding regimen was continued. Failure to reach >500 mL/day of EN by day 3 was an indication for discontinuation of the study. Patients who received >500 mL/day EN who also received supplemental PN or those not tolerating EN who received PN temporarily in order to meet their daily requirements after the first 3 days were not excluded. EN was continued until ICU discharge, death or completion of 28 days of the study.
Baseline characteristics, including age, sex, weight, height, body mass index, admission diagnosis, presence of head trauma, Glasgow Coma Scale (GCS), Acute Physiology and Chronic Health Evaluation II (APACHE II) score, Sequential Organ Failure Assessment (SOFA) score, ISS and New Injury Severity Score (NISS) were recorded at enrollment. Arterial blood gases (AVL system; Omni Technology, Graz, Austria) and the PaO2 (partial pressure of oxygen in arterial blood)/FIO2 ratio were routinely assessed every morning. Data for the study were collected on days 1, 4 and 8.
Analysis of red blood cell membrane fatty acid composition
Extraction of red blood cell (RBC) lipids, transmethylation and gas chromatographic analysis were performed as described previously by Green and Yavin with minor modifications [17]. Values were expressed as the percentage of a given fatty acid with respect to total identified fatty acids. The Omega-3 Index (O-3 I) was calculated by adding together the EPA and docosahexaenoic acid (DHA) percentages for each sample. The lipid membrane composition of RBCs and the O-3 I were measured on days 1, 4 and 8.
Treatment protocol
Patients were treated according to EAST trauma guidelines [18]. The mode of mechanical ventilation was left to the discretion of the attending physician. In all cases, the goals of mechanical ventilation included maintaining a peripheral capillary oxygen saturation (SpO2) of ≥ 90 %, peak airway pressure of <35 cmH2O and a tidal volume of <7 mL/kg. Levels of positive end-expiratory pressure. and FIO2 were adjusted according to the ARDS net protocol [19]. Decisions on the timing of extubation were left to the discretion of the attending physician.
Study outcomes
Primary outcomes
The primary outcomes were the level of oxygenation as assessed by the PaO2/FiO2 (PF) ratio on days 4 and 8 after admission, the incidence of ALI and/or ARDS according to the American–European Consensus Conference (AECC) definition (since the study was planned before the Berlin definition) [20] and length of ventilation.
Secondary outcomes
Secondary outcomes included: (1) the incidence of new organ failure as measured by the daily SOFA score (deterioration of existing organ dysfunction was not included as an adverse outcome); (2) the rate of new infections including wound infections [21], bacteremia, ventilator-associated pneumonia (VAP) [20]; (3) length of ICU stay; (4) length of hospital stay; (5) 28-day mortality.
Statistical analysis
All analyses were conducted in both an intention-to-treat (ITT) and a per-protocol population. For the per-protocol analysis, we excluded patients who received mechanical ventilation for <48 h or who were intolerant of EN. The PF ratio was collected only for patients who continued to require mechanical ventilation. The composition of the RBC membrane was analyzed only for patients who completed day 8 and for whom all three values (days 1, 4 and 8) were available for analysis.
An a priori power analysis was performed. In order to detect a statistically significant difference in any worsening of oxygenation on days 4 and 8 of the study, at least 60 patients in each group were needed for a power (1−β) of 0.9 and x of 0.05. Data are presented as mean values ± standard deviation (SD). Statistical analyses of clinical and metabolic status were performed with SPSS version 12.0 software (SPSS Inc., Chicago, IL) for Windows. Statistical analysis of the lipid membrane composition was performed with repeated-measures analysis of variance (ANOVA) and Tukey’s Multiple Comparison Post Test using Graphpad Prism 5.0 software (Graphpad Software Inc., La Jolla, CA). Differences between the study and control groups were calculated from baseline to day 8 with Student’s t test or one-way ANOVA for all continuous variables. For multiple comparisons, one-way ANOVA or repeated-measures ANOVA were used. Patients with isolated head trauma were included both in the overall analysis and analyzed separately as was planned in advance of the study close-out. All P values were two-sided, and significance was assigned at a threshold of <0.05. ANOVA for repeated measures was used to compare length of ventilation (LOV) and length of stay in the ICU, as well as oxygenation and lipid membrane composition.
Results
Patient enrollment
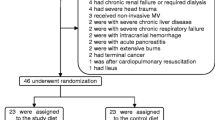
A total of 195 patients were screened, and 120 of these were initially enrolled in the study to form the ITT population. Of these 120 patients, 21 were subsequently excluded (16 due to early extubation and 5 due to failure to reach >500 cc EN by day 3). Thus, the per-protocol analysis included 99 patients, of whom 47 formed the control group (10 patients were excluded due to early extubation and 1 due to EN intolerance) and 52 formed the study group (6 patients were excluded due to early extubation and 4 due to EN intolerance). A study flow chart of the enrollment process is shown in Fig. 1.
Baseline characteristics
In both the ITT and per-protocol populations there were no significant differences between the two groups at baseline, with the exception of the number of patients suffering from isolated head trauma [ITT analysis: 22.4 (control group) vs. 8.0 % (study group), P < 0.04; per-protocol analysis: 23.4 (control group) vs. 3.8 % (study group), P < 0.007] (see "Appendix" Table 5).
Caloric and metabolic data
In the ITT population, mean daily REE was not significantly different between the control and study groups (2,205.9 ± 455.2 vs. 2,135.2 ± 410.8 kcal/day, respectively; P = 0.38) (Table 1). The amount of calories provided by EN and total calories received by the patient by EN, PN and other sources were also similar in both groups. Due to intolerance to EN, PN was given temporarily to five patients in the control group and six patients in the study group. There were no significant differences in mean daily glucose levels or in daily insulin requirements between the two groups. Tolerance to enteral feeding was not significantly different between the control and study groups as evidenced by the lack of a significant difference in number of GRV episodes [GRV of 150–500 mL: 3.2 ± 5.8 (control group) vs. 3.6 ± 5.1 episodes (study group), P = 0.70; GRV of >500 mL: 1.6 ± 4.4 (control) vs. 1.1 ± 2.3 episodes (study group), P = 0.42]. EN reached at least 80 % of the target in both groups. Similar non-significant differences were found for these data in the per-protocol analysis.
Primary outcome measures
In the ITT analysis, ventilatory parameters were not significantly different in the two groups [PEEP: 5.8 ± 1.1 (control group) vs. 5.7 ± 1.0 cm H2O (study group), P = 0.60; tidal volume: 540 ± 0.07 (control group) vs. 530 ± 0.07 mL (study group), P = 0.80]. The baseline PF ratio was not significantly different between the control and study groups (261.1 ± 103.7 vs. 266.0 ± 109.3, respectively; P = 0.80) (Table 2). In addition, no significant differences were found in the PF ratio between the control and study group on day 4 (213.7 ± 85.6 vs. 227.2 ± 67.8, respectively; P = 0.24) or on day 8 (187.8 ± 65.2 vs. 188.9 ± 56.0, respectively; P = 0.82) or in the change in oxygenation from baseline to day 4 and to day 8 [change from baseline to day 4: 46.7 ± 19.6 (control group) vs. 33.4 ± 33.4 (study group), P = 0.24; change from baseline to day 8: 66.5 ± 35.3 (control group) vs. 60.4 ± 53.1 (study group), P = 0.81] (Table 2). No significant differences were found between the control and study groups in the incidence of ALI/ARDS (24.1 vs. 29.0 %, respectively; P = 0.68) or in the LOV (13.6 ± 10.7 vs. 17.0 ± 15.1 days, respectively P = 0.15) (Table 2).
In the per-protocol analysis, ventilatory parameters were not significantly different in the two groups [PEEP: 5.8 ± 1.1 (control group) vs. 5.7 ± 1.0 cm H2O (study group), P = 0.69; tidal volume: 540 ± 0.07 (control group) vs. 530 ± 0.07 mL (study group), P = 0.57]. The baseline PaO2/FiO2 ratio was not significantly different between the control and study groups (263.4 ± 104.3 vs. 242.9 ± 97.7, respectively; P = 0.31) (Table 2). In addition, no significant differences were found in the PaO2/FiO2 ratio between the control and study group on day 4 (216.4 ± 84.4 vs. 221.3 ± 66.4, respectively; P = 0.35) or on day 8 (187.8 ± 65.2 vs. 187.9 ± 56.4, respectively; P = 0.94) or in the change in oxygenation from baseline to day 4 and to day 8 respectively [change from baseline to day 4: 47.0 ± 19.9 (control group) vs. 21.6 ± 31.3 (study group), P = 0.22; change from baseline to day 8: 66.5 ± 35.3 (control group) vs. 55.8 ± 47.9 (study group), P = 0.67) (Table 2). No significant differences were found in the incidence of ALI/ARDS between the control and study groups (27.7 vs. 34.6 %, respectively; P = 0.52) or in the LOV (16.4 ± 9.9 vs. 19.4 ± 15.2 days, respectively; P = 0.26) (Table 2).
Secondary outcomes
No significant differences between the two groups were found in the ITT or per-protocol analyses for secondary outcomes, including incidence of VAP (P = 0.45 and P = 0.41, respectively), wound infections (P = 0.81 and P = 1.0, respectively), incidence of new organ failures (P = 0.27 and P = 0.31, respectively), length of ICU stay (P = 0.21 and P = 0.32, respectively), hospital stay (P = 0.14 and P = 0.30, respectively) and 28-day mortality (P = 0.56 and P = 0.53, respectively) (Table 3).
In both the ITT and per-protocol analysis, the incidence of bacteremia was found to be significantly higher in the study group [3 (control group) vs. 14 (study group); P = 0.008 for ITT analysis and P = 0.03 for per-protocol analysis] (Table 3). In addition, study patients received significantly more packed RBC transfusions than control patients (189 vs. 77 units, respectively; P = 0.03). When patients with isolated head trauma was analyzed separately, no significant differences were noted between the two groups for any of the parameters studied.
RBC lipid membrane composition
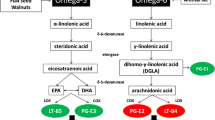
The study group showed a significant increase in both EPA and GLA concentrations at day 4 (P = 0.05) and at day 8 (P < 0.001) when compared to baseline (Fig. 2). No changes in any of the fatty acids studied were noted in the control group apart from a significant decrease in the DHA concentration on day 4. The omega-6 fatty acid profile remained unchanged in the control group. The O-3 I increased significantly in the study group from 5.5 ± 1.5 at baseline to 6.0 ± 1.2 and 6.8 ± 1.4 at day 4 and day 8, respectively (P < 0.005) but remained unchanged in the control group (Table 1).
Discussion
To our knowledge, our study is the first to investigate the effect of the continuous and preemptive administration of EPA, GLA and antioxidants in a homogeneous group of ventilated, critically ill patients with multiple trauma admitted to the ICU. No significant differences between these patients and those receiving a control formula were found in terms of oxygenation, incidence of ALI or ARDS and LOV.
Previous studies, including those from our center, have shown improved oxygenation in critically ill patients with established ALI or ARDS receiving continuous nutritional support with fish oil [5–7]. We postulated that the preemptive provision of EPA, GLA and antioxidants may have the potential to blunt the inflammatory response characteristic of severe trauma and thus limit respiratory complications. However, we were unable to demonstrate any benefit of the study formula over the control formula in any of the primary respiratory endpoints, including changes in oxygenation from admission to the ICU, incidence of new-onset ALI or ARDS or length of mechanical ventilation. While these results suggest that EN-supplemented fish oil may not have a beneficial effect on these parameters, it also is possible that our negative results may be related to the dose of fish oil delivered to each patient. Therefore, we measured the fatty acid membrane composition of the RBC. EN enriched in omega-3 fatty acids has been shown to alter the fatty acid composition of cells, including those involved in inflammatory responses, such as neutrophils and macrophages [23]. In this context, it has been suggested that the O-3 I, which is the combined EPA + DHA content of erythrocytes expressed as a percentage of total identified fatty acids, may provide important and independent information on the health status of, for example, patients with cardiovascular disease [24]. An optimal O-3 I of >8 % has been defined for cardiovascular risk. Thus, it is possible to detect omega-3 deficits and assess the adequacy of supplementation. We have recently that the RBC membrane of trauma patients has a low content of total n-3 fatty acids compared to that of healthy volunteers (7.5 ± 0.2 vs. 11.9 ± 0.2 %, respectively [25]). In the present study, we demonstrated that EPA, DHA and GLA were incorporated into the membrane from day 4 onwards, although the O-3I values failed to reach those suggested as being optimal, at least for cardiac patients, to obtain clinical efficacy. Optimal levels have as yet not been evaluated in critically ill patients. However, supplementation with larger amounts of fish oil may be required to achieve a positive clinical effect. Consequently, additional studies need to be performed in which the amount of supplementation is targeted to a potentially measurable endpoint, i.e. the O-3 I, and levels correlated with clinical outcomes.
With respect to the LOV, a recent study evaluating the effect of a fish oil/antioxidant combination given at the onset of sepsis or systemic inflammatory response syndrome (SIRS) as a continuous infusion found that patients in the study group required less mechanical ventilation [26]. However <50 % of patients in this study were mechanically ventilated so that no direct comparison can be made with our study, where no differences were noted between the two groups.
No significant differences between the groups were noted in the incidence of secondary outcomes, which were related to the development of infectious complications either in the ITT or per-protocol analysis. However, a significantly higher incidence of bacteremia was noted in the study group in both the ITT and per-protocol analysis. This may be explained by the significantly higher number of RBC transfusions received by patients in the study group compared to the control group, due to the larger number of patients in the study group with multiple trauma compared to isolated head trauma; RBC transfusions are a known risk factor for bacteremia [27]. No effect of supplementation on any of the other outcome measures was noted when isolated head trauma patients were removed from the statistical analysis.
It has been suggested that the positive effects reported in previous studies [5–7] were not linked to the presence of EPA and GLA but to the deleterious effects of large amounts of long chain triglycerides (LCTs) [28–30] received by patients in the control groups. Our study did not show an increase in morbidity and/or mortality in the patients of the control group receiving large amounts of LCTs and therefore does not support this hypothesis. Our results also suggest that a diet enriched in omega-6 lipids, such as that received by the control group, did not modify the membrane in a deleterious way, since arachidonic acid composition was not altered (Fig. 1).
There were a number of limitations to the study. First, while no effects on the primary outcome measures were found, it should be noted that the per-protocol analysis was underpowered according to the power analysis calculation. Secondly, there were more patients with isolated head trauma in the study group than in the control group. However, since patients suffering from isolated head trauma were included sequentially in the overall randomization process (i.e. as a patient with multiple trauma or isolated head trauma), this result is coincidental. In addition, an analysis of the results excluding those with head trauma did not alter the results in either the ITT or per-protocol analysis. Thirdly, caloric intake achieved 89 and 87 % of measured REE in the study and control groups, respectively. While this reflects strict adherence to the protocol and the use of a nutritional bundle including a dedicated dietician, it should be noted that 21 patients were excluded from the study, as previously described. Finally, we cannot exclude an effect of RBC transfusions on the red cell lipid membrane composition for which we had results for all three time-points in 33 control patients and 40 study patients.
These results also stress the importance of measuring individual requirements for each nutrient when nutrients are administered as supplementation in critically ill patients, as has been suggested following the administration of high doses of glutamine [31, 32] and selenium [33], as well as in the study conducted by Rice et al. [10]. Importantly, concentrations of nutrients may vary according to the underlying disease, the region and dietary habits and may be further modified by the mode of administration (dose and bolus or continuous).
In conclusion, in our study the administration of EPA and GLA did not result in improved outcomes in mechanically ventilated patients with multiple trauma despite their demonstrated integration into the membrane of RBCs. These results may be related to the lack of an anti-inflammatory effect of the EPA and GLA administered in patients having very low baseline levels. Therefore, in this specific population, we cannot recommend this specialized formula at this dosage. Studies titrating the dose of EPA and GLA in specific populations with the aim of determining an effective dosage should be planned.
References
Giannoudis PV (2003) Current concepts of the inflammatory response after major trauma: an update. Injury 34:397–404
Doig GS, Heighes PT, Simpson F, Sweetman EA, Davies AR (2009) Early enteral nutrition, provided within 24 h of injury or intensive care unit admission, significantly reduces mortality in critically ill patients: a meta-analysis of randomised controlled trials. Intensive Care Med 35:2018–2027
Kreymann KG, Berger MM, Deutz NE, Hiesmayr M, Jolliet P, Kazandjiev G et al (2006) ESPEN guidelines on enteral nutrition: intensive care. Clin Nutr 25:210–223
McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, Ochoa JB, Napolitano L, Cresci G (2009) Guidelines for the provision and assessment of nutrition support therapy in the adult critically III patient: society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N). JPEN J Parenter Enteral Nutr 33:277–316
Gadek JE, DeMichele SJ, Karlstad MD, Pacht ER, Donahoe M, Albertson TE, Van Hoozen C, Wennberg AK, Nelson JL, Noursalehi M (1999) Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Enteral Nutrition in ARDS Study Group. Crit Care Med 27:1409–1420
Singer P, Theilla M, Fisher H, Gibstein L, Grozovski E, Cohen J (2006) Benefit of an enteral diet enriched with eicosapentaenoic acid and gamma linolenic acid in ventilated patients with acute lung injury. Crit Care Med 34:1033–1038
Pontes-Arruda A, Araga˜o AM, Albuquerque JD (2006) Effects of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in mechanically ventilated patients with severe sepsis and septic shock. Crit Care Med 34:2325–2333
Todd SR, Kozar RA, Moore FA (2006) Nutrition support in adult trauma patients. Nutr Clin Pract 21:421–429
Stapleton RD, Martin TR, Weiss NS et al (2011) A phase II randomized placebo-controlled trial ofomega-3 fatty acids for the treatment of acute lung injury. Crit Care Med 39:1655–1662
Rice TW, Wheeler AP, Thompson BT, deBoisblanc BP, Steingrub J, Rock P, NIH NHLBI Acute Respiratory Distress Syndrome Network of Investigators (2011) Enteral omega-3 fatty acid, and linolenic acid, and antioxidant supplementation in acute lung injury. JAMA 12(306):1574–1581
Zhu D, Zhang Y, Li S, Gan L, Feng H, Nie W (2014) Enteral omega-3 fatty acid supplementation in adult patients with acute respiratory distress syndrome: a systematic review of randomized controlled trials with meta-analysis and trial sequential analysis. Intensive Care Med 40:504–512
Andrews PL, Shiber JR, Jaruga-Killeen E, Roy S, Sadowitz B, O'Toole RV et al (2013) Early application of airway pressure release ventilation may reduce mortality in high-risk trauma patients: a systematic review of observational trauma ARDS literature. J Trauma Acute Care Surg 75:635–641
Raymondos K, Martin MU, Schmudlach T, Baus S, Weilbach C, Welte T, Krettek C, Frink M, Hildebrand F (2012) Early alveolar and systemic mediator release in patients at different risks for ARDS after multiple trauma. Injury 43:189–195
Singer P, Doig GS, Pichard C (2014) The truth about nutrition in the ICU. Intensive Care Med 40:252–255
Weirs J (1949) New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109:1
Faisy C, Guerot E, Diehl JL, Labrousse J, Fagon JY (2003) Assessment of resting energy expenditure in mechanically ventilated patients. Am J Clin Nutr 78:241–249
Green P, Yavin E (1996) Fatty acid composition of late embryonic and early postnatal rat brain. Lipids 31:859–865
Pasquale M, Fabian T (1998) Practice management guidelines for trauma from the Eastern Association for the surgery of trauma. J Trauma 44:941–956
Acute Respiratory Distress Syndrome Network (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L et al (1994) The American–European Consensus Conference on ARDS definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149:818–824
Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG (1992) CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 13:606–608
Johanson Gaynes RP, Horan TC (1999) Surveillance of nosocomial infections. In: Mayhall CG (ed) Hospital epidemiology and infection control. Lippincott Williams & Wilkins, Philadelphia, pp 1285–1317
Calder PC (2010) Omega-3 fatty acids and inflammatory processes. Nutrients 2:355–374
Harris WS (2010) The omega-3 index: clinical utility for therapeutic intervention. Curr Cardiol Rep 12:503–508
Silva V, Green P, Singer P (2014) Membrane fatty acid composition of different target populations: importance of baseline on supplementation. Clin Nutr 33:206
Pontes-Arruda A, Martins LF, de Lima SM et al (2011) Investigating nutritional therapy with EPA, GLA and antioxidants role in sepsis treatment (INTERSEPT) Study Group. Enteral nutrition with eicosapentaenoic acid, γ-linolenic acid and antioxidants in the early treatment of sepsis: results from a multicenter, prospective, randomized, double-blinded, controlled study: the INTERSEPT study. Crit Care 15:R144. doi:10.1186/cc10267
Shorr AF, Jackson WL, Kelly KM, Fu M, Kollef MH (2005) Transfusion practice and blood stream infections in critically ill patients. Chest 127:1722–1728
Preiser JC (2008) Nutrition therapy for acute respiratory distress syndrome. JPEN J Parenter Enteral Nutr 32:669–670
Krzak A, Pleva M, Napolitano LM (2011) Nutrition therapy for ALI and ARDS. Crit Care Clin 27:647–659
Parshuram C, Kavanagh BP (2004) Positive clinical trials: understand the control group before inplementimg the result. Am J Respir Crit Care Med 170:223–226
Heyland D, Muscedere J, Wischmeyer PE, Canadian Critical Care Trials Group et al (2013) A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med 368(16):1489–1497
Alhazzani W, Jacobi J, Sindi A, Hartog C, Reinhart K, Kokkoris S et al (2013) The effect of selenium therapy on mortality in patients with sepsis syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care Med 41(6):1555–1564
Pérez-Bárcena J, Marsé P, Zabalegui-Pérez A, Corral E, Herrán-Monge R, Gero-Escapa M et al (2014) A randomized trial of intravenous glutamine supplementation in trauma ICU patients. Intensive Care Med 40:539–547
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Take-home message: This article describes the effects of the administration of high doses of fish oil (EPA) and gamma-linolenic acid as a preemptive treatment to decrease the incidence of ARDS and improve oxygenation. The findings of the study are interesting since it is the first time these nutrients have been administered in a preemptive manner to such a selected population (ventilated patients with multiple trauma) and with the analysis of fatty acid membrane composition. No significant clinical effect was observed, but the fact that the membrane composition was only mildly affected may explain the clinical findings and encourage monitoring of these parameters to achieve a clinical effect.
Rights and permissions
About this article
Cite this article
Kagan, I., Cohen, J., Stein, M. et al. Preemptive enteral nutrition enriched with eicosapentaenoic acid, gamma-linolenic acid and antioxidants in severe multiple trauma: a prospective, randomized, double-blind study. Intensive Care Med 41, 460–469 (2015). https://doi.org/10.1007/s00134-015-3646-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-015-3646-z