Abstract
Purpose
The aim of the present study was to assess the rate of diffuse alveolar damage (DAD) on open lung biopsy (OLB) performed in the ICU for nonresolving ARDS.
Methods
A single-center retrospective study of patients meeting the Berlin definition criteria for ARDS who had undergone OLB for nonresolving ARDS. Patients were classified into mild, moderate and severe ARDS categories and according to the presence or absence of DAD on the OLB. The ARDS categories were assessed at baseline and at the time of the OLB. The OLBs were reviewed by two pathologists blinded to the ARDS classification. The primary endpoint was the rate of DAD according to the ARDS stage in the patients with nonresolving ARDS who had OLB. The secondary endpoint was the ability of DAD to predict ARDS among all the patients who had OLB. The same clinico-histopathological confrontation was cross validated in another ICU.
Results
From January 1998 to August 2013, 113 patients underwent OLB for acute hypoxemic respiratory failure, 83 of whom met the inclusion criteria for ARDS. At the time the OLB was performed, 11 of these patients had mild, 56 moderate, and 16 severe ARDS, respectively. The median (1st–3rd quartiles) time to OLB was 13 (10–18) and 9 (6–14) days from the onset of respiratory symptoms and from ARDS onset, respectively, with no statistical difference between the three ARDS groups. DAD was found in 48 (58 %) patients with ARDS, 4 (36 %) in the mild, 33 (59 %) in the moderate, and 11 (69 %) in the severe stage (P = 0.23). For the 113 patients who underwent OLB, the sensitivity and specificity of DAD to the Berlin definition was 0.58 (0.46–0.69) and 0.73 (0.54–0.88), respectively. Similar results were found in the other ICU.
Conclusions
DAD is present in the majority of patients with nonresolving ARDs and its frequency is no different across the three ARDS stages. On this basis, the systematic use of steroids in nonresolving ARDS is not recommended.
Similar content being viewed by others
Introduction
Diffuse alveolar damage (DAD) is a typical pathological finding in acute respiratory distress syndrome (ARDS) [1] and is commonly thought of as its pathological hallmark [2–4]. This is a controversial statement, however, as DAD is commonly seen in other pathologies. In the initial description of ARDS by Asbaugh et al. [5], not all patients had DAD. Some experts have suggested that, as long as the clinical criteria for ARDS are met, pneumonia without DAD may be a suitable histologic correlate for ARDS. Open lung biopsies (OLB) can be performed in ARDS patients at the ICU bedside [6]. When the procedure is carried out by trained ICU teams, the reported level of safety is satisfactory [7]. In one study, when OLB was included in patient management, outcomes significantly improved [7]. In nonresolving ARDS, OLB can be indicated to identify either disease-specific lung involvement (systemic disease with an acute presentation) or the presence of DAD. This is important for the use of steroids. If DAD is frequent in nonresolving ARDS, the systematic use of steroids may not be appropriate. Indeed, in a randomized controlled trial of steroids versus placebo for ARDS, starting steroids after 2 weeks of ARDS was associated with higher mortality [8]. It should be noted that there are very few data available on OLB in nonresolving ARDS.
A group of experts recently proposed the Berlin definition for ARDS [9, 10]. Under the Berlin proposal, (1) the acute timeframe is defined as within 1 week after the onset of a known clinical injury and new or worsening respiratory symptoms; (2) the assessment of oxygenation takes into account positive end-expiratory pressure (PEEP), which must be at least 5 cmH2O at the time of the ARDS definition; (3) patients are split into mild, moderate, and severe categories based on the severity of hypoxemia.
The present study was based on our extensive experience with OLB in patients with acute hypoxemic respiratory failure, the rather limited data available in the literature regarding OLB in this setting, and the lack of any previous confrontation between OLB and the new criteria for defining ARDS.
Our primary goal was to assess the rate of DAD in patients with nonresolving ARDS across the different ARDS stages. Our hypothesis was that DAD was more frequently observed in the severe ARDS stage than in the other two categories. This assumption was subtended by the 69 % rate of DAD in patients with severe ARDS that had developed for more than 72 h at the time of autopsy [11].
Methods
Authorization to report the present results was obtained from the ethics committee (Comité Consultatif de Protection des personnes Sud-Est II on 10 October 2013, CAL 2013-041). Informed consent from the patients or their next of kin was waived in accordance with French law.
Patients
We reviewed the charts of all the patients who underwent OLB in our 15-bed medical ICU between 1 January 1998 and 31 August 2013 for the diagnostic evaluation of acute hypoxemic respiratory failure. Patients with OLB were split into an ARDS group, which included those patients who met the Berlin criteria for ARDS at any time between ICU admission and OLB, and a non-ARDS group. Patients were included in the latter group if any of the following criteria were met: (1) more than 1 week had elapsed from the onset of new or worsening respiratory symptoms after a recognized lung insult; (2) PEEP of less than 5 cmH2O (unless the patient was first identified as having ARDS at the time of ICU admission but subsequently had to receive PEEP < 5 cmH2O as a result of reduced lung compliance; in this case the patient remained in the ARDS group but the severity was still assessed according to oxygenation); (3) PaO2/oxygen fraction in the inspired air (FiO2) < 300 mmHg. The number of patients with acute respiratory failure admitted to our ICU during the study period was also retrieved (with the international classification of diseases and related health problems ICD-10 codes J80 or J81 or J96 encoded as the primary or related or significantly associated diagnosis).
Open lung biopsy
OLB was indicated for persistent acute hypoxemic respiratory failure with bilateral lung opacities more than 7 days after ICU admission or weaning failure from invasive mechanical ventilation or NIV after having ruled out cardiogenic causes and ongoing lung infection. The latter was established from the absence of microorganisms in the broncho-alveolar lavage (BAL) fluid culture performed 2–3 days before the OLB was scheduled. The OLB was thus indicated to determine the origin of persistent lung failure. The ICU physicians and the surgeon gave their joint agreement to carry out the OLB. It should be acknowledged that overall we were not able to state that all the patients with an OLB indication actually underwent the procedure.
Depending on the severity of hypoxemia and local conditions, the OLB was performed either at the bedside or in theater by a surgeon trained in thoracic surgery, using the technique introduced by Papazian et al. [6]. If the PaO2/FiO2 ratio < 150 mmHg with FiO2 > 60 % and PEEP > 10 cmH2O, the OLB was performed at the bedside. In the event of coagulation abnormalities and/or expected local difficulties based on clinical history and thoracic computed tomography (CT) findings the procedure was performed in the operating theater. The side on which the lung sample was taken was determined by the CT scan. The patient was placed in a lateral position on the opposite side from that where the thoracotomy was performed. During the procedure the patient was sedated, paralyzed, and ventilated in volume assist controlled mode with the same settings as those used prior to the OLB. The largest lung sample possible, up to 2 cm in length, was removed and cut into smaller pieces for microbiological cultures (bacteriology, mycology, and virology) and pathology. The pleural space was drained using a single chest tube or two chest tubes if the patient was scheduled for further prone positioning sessions. The safety of the OLB was assessed based on the record of air leaks and bleeding during the first week following the procedure.
For OLB processing, see the ESM.
Defining groups
Lung pathology grouping
The histological findings were divided into the DAD and non-DAD groups. The DAD group included DAD in the acute (or exudative) phase (defined as the presence of edema, hyaline membrane lining alveoli, and interstitial acute inflammation) and DAD in the organizing (or proliferative) phase (defined as the presence of organizing fibrosis, usually diffuse, mostly within the alveolar septa,) or DAD with an airspace organization and type II pneumocyte hyperplasia or honeycomb fibrosis. The non-DAD group included interstitial lung fibrosis (ILF) due to the acute exacerbation of idiopathic pulmonary fibrosis, defined as exudative phase DAD superimposed on underlying usual interstitial pneumonia (UIP) [12]; or other patterns of lung fibrosis [12]; or organizing pneumonia (OP) defined as patchy involvement of alveolar ducts and alveoli with or without bronchiolar intraluminal polyps, centered on small airways, without a hyaline membrane or diffuse pattern; or edema and thickening of the alveolar walls as seen in organizing DAD or bacterial pneumonia defined by the presence of scattered neutrophilic infiltrates localized to terminal bronchioles and surrounding alveoli with evident confluence of infiltrates between adjacent lobules [16]; or other patterns. Two pathologists blinded to the ARDS classification reviewed all the samples for the purposes of the present study.
ARDS grouping
The patient charts were thoroughly reviewed by two authors to identify the nature of the clinical insult, the time of onset of the respiratory symptoms, the chest X-ray, and the cardiogenic involvement in the lung edema. In the event of disagreements the charts were reviewed for consensus. The clinicians were blinded to the pathology results when they reviewed the charts. In the ARDS patients included based on the criteria listed above (baseline), the ARDS criteria were reassessed on the day of the OLB. At each assessment, the ARDS stages were defined as follows: mild ARDS was defined as a PaO2/FiO2 ratio of between 300 and 201 mmHg under PEEP or continuous positive airway pressure (CPAP) ≥ 5 cmH2O; moderate ARDS as a PaO2/FiO2 ratio between 200 and 101 mmHg under PEEP ≥ 5 cmH2O; and severe ARDS as a PaO2/FiO2 ratio of below 100 mmHg under PEEP ≥ 5 cmH2O.
Data collection
The following data were recorded at the time of ICU admission: age, gender, reason for ICU admission, and comorbidities according to Charlson [13], Simplified Acute Physiology Score (SAPS) II [14], predicted body weight [15], body mass index (BMI), and Sequential Organ Failure Assessment (SOFA) score [16]. SOFA and ventilator settings [tidal volume (VT), FiO2, PEEP, breathing frequency] were recorded at baseline and at the time of OLB. The date of the OLB and whether it was performed in the ICU or in the operating theater was recorded. Patients were followed up to ICU discharge, and whether they were living or dead at discharge was recorded. The cause of death was summarized as hypoxemia, multiple organ failure, refractory shock, cardiac arrest, or other according to the final medical report. Furthermore, information on whether an end-of-life decision was made during the ICU stay was also recorded.
Data analysis
Values were expressed as the median (first-third quartiles) for continuous values and counts (per percent of group) for qualitative variables. The normal distribution was checked for continuous variables using the Shapiro-Wilk test. The diagnostic performance of OLB was defined in terms of sensitivity, specificity, and negative and positive predictive values, together with their 95 % confidence intervals, against the Berlin definition. The disease was ARDS (yes/no), and the diagnostic test was DAD (yes/no).
The primary endpoint was to compare the rate of patients with DAD across the three ARDS stages. Secondary endpoints were the capability of DAD to predict ARDS among all the patients who had undergone OLB (those with and those without ARDS) and patient outcomes.
In order to assess the internal validity of our study we performed a case-control study in our ICU by matching ARDS patients who had OLB (cases) with ARDS patients who did not have OLB (ARDS controls) in the same period for age ±5 years, gender, and SAPSII ±7.
In order to assess the external validity of our study, the charts of patients who had undergone OLB for acute respiratory failure in another ICU (the medical ICU of the University Hospital in Clermont-Ferrand, France) were reviewed for the same variables as above by two investigators blinded to the lung pathology result. The OLBs in the other ICU were also reviewed by two pathologists blinded to the ARDS stage.
Comparisons between groups were performed using parametric or nonparametric tests as required. The comparison of the distribution of histopathological findings across the ARDS categories was carried out using Mantel-Haenszel chi-square test.
The statistical analysis was performed using R software (R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, URL http://www.R-project.org). The statistically significant threshold was set to P < 0.05.
Results
ARDS groups
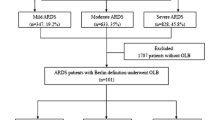
From 1 January 1998 to 30 June 2013, 905 patients were admitted to our ICU with a primary diagnosis of acute hypoxemic respiratory failure, of whom 597 had ARDS (Fig. 1; Table 1 ESM). OLBs were performed on 113 patients: 30 with no ARDS criteria and 83 with ARDS criteria (Fig. 1). Out of the 83 ARDS patients who underwent OLB, 8 had mild ARDS, 44 moderate ARDS, and 31 severe ARDS at baseline assessment. Only three patients, all with mild ARDS, were under noninvasive ventilation at the time of ICU admission. These patients were subsequently intubated. The remaining 80 patients with OLB and ARDS were intubated at the time of ICU admission. Baseline variables did not differ across the three groups (Table 2 ESM). For the 83 patients with ARDS and OLB, the time to OLB was 13 (9–16), 12 (10–18), and 14 (11–18) days (P = 0.50) from the onset of respiratory symptoms and 10 (7–16), 8 (5–14), and 10 (7–16) days (P = 0.26) from ARDS onset for the mild, moderate, and severe groups, respectively.
At the time of OLB, 11, 56, and 16 patients had mild, moderate, and severe ARDS, respectively, with no significant differences among the three ARDS groups in terms of SOFA score, ventilator settings, and plateau pressure (Table 3 ESM).
Primary endpoint: distribution of ARDS stages according to DAD on OLB
Out of the 83 ARDS patients who underwent OLB, DAD was found in 48 patients (57.8 %). The proportion of patients with DAD was not different among the three ARDS groups at either ARDS assessment (Table 1) even when the mild and moderate categories were merged and compared with the severe ARDS category (Fig. 2). At the time of OLB, the frequency of mild, moderate, and severe ARDS stage was 8, 69, and 23 % in cases of DAD versus 20, 66, and 14 %, respectively, in the absence of DAD.
Patients with DAD were significantly more hypoxemic and had higher plateau pressure for similar PEEP and VT than patients without DAD (Table 1).
The diagnostic accuracy of the Berlin definition for DAD in patients with nonresolving ARDS
In the 30 patients who did not have ARDS who underwent OLB, 8 had DAD and 22 did not (Table 2). Therefore, for the 113 patients who underwent OLB in our ICU, the sensitivity and specificity of DAD to predict ARDS from the Berlin definition were 0.58 (0.46–0.69) and 0.73 (0.54–0.88), respectively, in patients with nonresolving ARDS. The negative and positive predictive values were 0.39 (0.26–0.52) and 0.86 (0.74–0.94), respectively.
ARDS cases versus controls
The 83 patients with ARDS and OLB (cases) were matched with 83 patients with ARDS and no OLB (ARDS controls) (Table 4 ESM). There was no significant difference between the two groups in terms of baseline confounders except for the cause of ARDS (Table 3). The reasons for not performing OLB in the control group were resolving ARDS within 1 week (45 patients), death before OLB (9), OLB contraindicated (3), end-of-life decision (7), ongoing steroids and no change in management expected from OLB (6), steroids contraindicated (5), other reasons for the persistent hypoxemia (2), and undetermined (6).
External validity
The same confrontation between OLB and ARDS clinical definitions was performed in another ICU. The reason for this was to assess the results in our ICU for both consistency and generalizability. Out of the 32 patients with OLB in the other ICU (Fig. 1 ESM), 22 had ARDS criteria (13 with DAD and nine without DAD) and 10 did not (six with DAD and four without DAD). DAD was present in 19 patients, giving a prevalence of 59 %, as in our ICU (P = 1.00 Fisher exact test). For the variables listed in Table 1, the 22 patients with ARDS did not differ from the 83 ARDS patients in our ICU (not shown). The sensitivity and specificity of DAD to predict ARDS were 0.59 (0.36–0.79) (not significantly different from that found in our ICU, P = 1.00 Fischer's exact test) and 0.40 (0.15–0.74) (P = 0.12), respectively. The negative and positive predictive values were 0.31 (0.09–0.61) and 0.68 (0.43–0.87), respectively.
OLB safety
No deaths were attributable to the OLB. Complications occurred in 21 patients (25 %): air leaks in 20 (11 leaky chest tubes without pneumothorax, 7 pneumothoraces requiring chest tubes, 1 subcutaneous emphysema without pneumothorax, and 1 bronchopleural fistula after chest tube removal) and bleeding in 1 patient.
Outcomes
In our ICU, ICU fatality and end-of-life decision rates were similar between the case and the ARDS control groups (Table 4 ESM). Hypoxemia was a more frequent cause of death in cases than in ARDS controls (Table 4 ESM). No difference was found across the three ARDS groups for the ICU fatality rate, end-of-life decision, and cause of death (Table 2 ESM). The same was true between our ICU and the other ICU (not shown).
Discussion
The main result of our study is that DAD is frequently observed in nonresolving ARDS regardless of its severity.
Relevance of the present study
Defining ARDS is of paramount importance for further improving patient management. The Berlin approach, like the previous American-European Consensus Conference definition, is indirect as it does not include basic pathophysiological impairments, such as inflammation, increased alveolar-capillary membrane permeability, and increased extravascular lung water. Previous studies have compared the clinical definition of ARDS to pathological autopsy findings [11, 17]. The novelty of the present study is that we focused on nonresolving ARDS to obtain lung pathological findings with therapeutic implications, in particular regarding the use of steroids when the patients were still alive.
Moreover, we found that the distribution of DAD was not different across the clinical ARDS categories in nonresolving ARDS. Similar results were obtained in the other ICU used for external validation. A basic assumption in our study is that DAD is the hallmark of ARDS. This common conception may be overstated as there are no absolute data to back it up. DAD is not uncommon in OLB performed for unilateral lung disease [18]. The prevalence of DAD in ARDS is 24 % in the literature [7, 19]. However, Parambil et al. [23] found that in 58 consecutive patients with OLB-proven DAD, 90 % of them met the ARDS criteria, further illustrating the controversy in this area.
A secondary endpoint of our study was to assess the diagnostic performance of DAD compared to the Berlin definition in patients with nonresolving ARDS. The operating ARDS definitions have been used in confrontations with lung histology in two autopsy studies. Esteban et al. [17] found that the previous definition of ARDS had 74 % sensitivity and 84 % specificity in 127 patients. When 284 patients with ARDS risk factors were analyzed, sensitivity was found to be 76 % and specificity 75 %. Thille et al. [11] extended the previous database [17] to 712 autopsies and found that the Berlin definition had 89 % sensitivity and 63 % specificity when predicting DAD. In our study, DAD predicted ARDS with a sensitivity of 58 % and specificity of 73 %. One reason for this lower figure could be the limitations of the OLB per se, as it only explores part of the lung as compared to an autopsy, and to the case mix, since autopsies are performed in patients with fatal outcomes who may not be representative of the whole ARDS spectrum. In previous studies on OLB in ARDS, the rates of DAD ranged between 7 [20] and 60 % [19], but most of these studies were performed in the early stage of ARDS [6, 19–22], making the comparison with the present study difficult.
The safety of OLB in ARDS patients in our study was satisfactory. One-fourth of patients had air leaks but only seven (8 %) required chest drainage for pneumothorax. Although a significantly higher number of deaths from hypoxemia was observed in the OLB group than in the control group, no deaths in the study were attributable to OLB. It should be noted that OLB was not included in the Berlin definition as it is a high-risk procedure and its noninclusion was considered not to affect its prognostic ability.
Limitations and strengths
The present study has several limitations. First, it is retrospective. Second, it is a single-center study. Third, there is an obvious selection bias in the present study for a variety reasons, the first being that nonresolving ARDS, as defined in present study, represents a small subset of the patients with ARDS recommended for OLB for different reasons. This subset is by definition not representative of the “whole” of all clinically diagnosed cases of ARDS. The second reason is that OLB was not performed on every patient with nonresolving ARDS in our ICU. The ICU mortality rate in the three ARDS groups clearly highlights this limitation. Although the intention was to perform OLB in every patient with nonresolving ARDS, we cannot categorically state that all patients with an OLB indication actually underwent the procedure. Indeed, in the control group where the reasons for not performing OLB were recorded, six patients had not undergone an OLB for undetermined reasons. Therefore, our present sample may not be representative of the population of nonresolving ARDS. To assess the magnitude of this selection bias we conducted a retrospective case-control study in our ICU. It should be noted that the baseline confounding factors were not significantly different between the ARDS cases and controls. We expected the ARDS cases to be associated with more comorbidities, more severe acute illness, and more severe ARDS. This was apparently not the case. Even though the patients in the present study are selected patients, the selection bias may be less important than in autopsy studies as they are alive.
The present study does, however, have several strengths. First, the review of the lung samples was double blinded, a matched case-control study was performed, and external validation of the results was carried out in another ICU. Second, the present results provide new data from a specific subpopulation of ARDS patients, enhancing the existing evidence in this field. It is of interest that in nonresolving ARDS the baseline clinical characteristics of patients were similar in our ICU to those in the external ICU. This would indicate that baseline characteristics do not predict nonresolution.
Clinical implications
The fact that the prevalence of DAD is not different between ARDS categories implies that patients in the mild category really have ARDS and hence should receive lung protective ventilation. This reinforces the fitting of ventilator interventions to ARDS categories [10] provided in the Berlin definition.
In patients with nonresolving ARDS the use of steroids may be considered after ongoing infection has been ruled out. A large randomized controlled trial failed to demonstrate improvements in patient survival with steroids as compared to placebo [8]. Given the prevalence of DAD found on OLBs in nonresolving ARDS in the present study, it is unlikely that steroids would improve lung function. The only pathological diagnosis that is steroid-sensitive in this setting, other than a specific ARDS-causing immunological disease, is organizing pneumonia. We found an uncommonly large number of patients with organizing pneumonia in our study (9/83 patients). Previous studies have found much lower rates, such as 1 out of 64 patients in the study by De Hemptinne et al. [24]. A similarly (13/83) uncommonly high rate of interstitial lung fibrosis was also observed in our study as compared to previous studies [11, 17, 24]. It could be that these findings pertained to post-DAD fibrosis given the time lag between ARDS diagnosis and OLB [25]. The same remark would also apply to organizing pneumonia. If these findings had been accurately identified on the lung CT scan [26] the OLB might not have been performed. It should be noted that it was sometimes difficult to distinguish between organizing pneumonia and DAD when the OLB was small with a limited amount of tissue. Diffuse rather than patchy lung involvement, conspicuous type II pneumocyte hyperplasia, interstitial thickening rather than purely intraluminal polypoid plugs, foci of hyaline membrane and fibrin, and vascular fibrin microthrombi were the histological features that indicated the organizing phase of DAD rather than an organizing pneumonia pattern per se.
It should be noted that a key conclusion from the data is that performing an OLB for nonresolving ARDS does not add any clinically relevant prognostic information as mortality was similar whether DAD was present or absent.
In conclusion, in the specific context of nonresolving ARDS, DAD is found in the majority of patients with ARDS with no statistically significant difference across the ARDS stages.
References
Tomashefski JF Jr (1990) Pulmonary pathology of the adult respiratory distress syndrome. Clin Chest Med 11:593–619
Albertine KH (1985) Ultrastructural abnormalities in increased-permeability pulmonary edema. Clin Chest Med 6:345–369
Albertine KH (1998) Histopathology of pulmonary edema and the acute respiratory distress syndrome. In: Matthay MA, Ingbar DH (eds) Pulmonary edema. Marcel Dekker, New York, pp 37–84
Bachofen M, Weibel ER (1982) Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med 3:35–56
Ashbaugh DG, Bigelow DB, Petty TL, Levine BE (1967) Acute respiratory distress in adults. Lancet 2:319–323
Papazian L, Thomas P, Bregeon F et al (1998) Open-lung biopsy in patients with acute respiratory distress syndrome. Anesthesiology 88:935–944
Papazian L, Doddoli C, Chetaille B et al (2007) A contributive result of open-lung biopsy improves survival in acute respiratory distress syndrome patients. Crit Care Med 35:755–762
Steinberg KP, Hudson LD, Goodman RB et al (2006) Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 354:1671–1684
Ranieri VM, Rubenfeld GD, Thompson BT et al (2012) Acute respiratory distress syndrome. The Berlin definition. JAMA 307:E1–E8
Ferguson ND, Fan E, Camporota L et al (2012) The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 38:1573–1582
Thille AW, Esteban A, Fernandez-Segoviano P et al (2013) Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. Am J Respir Crit Care Med 187:761–767
Raghu G, Collard HR, Egan JJ et al (2011) An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183:788–824
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
ARDSnet (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The acute respiratory distress syndrome network. N Engl J Med 342:1301–1308
Vincent JL, Moreno R, Takala J et al (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on Sepsis-Related problems of the European Society of intensive care medicine. Intensive Care Med 22:707–710
Esteban A, Fernandez-Segoviano P, Frutos-Vivar F et al (2004) Comparison of clinical criteria for the acute respiratory distress syndrome with autopsy findings. Ann Intern Med 141:440–445
Yazdy AM, Tomashefski JF Jr, Yagan R, Kleinerman J (1989) Regional alveolar damage (RAD). A localized counterpart of diffuse alveolar damage. Am J Clin Pathol 92:10–15
Patel SR, Karmpaliotis D, Ayas NT et al (2004) The role of open-lung biopsy in ARDS. Chest 125:197–202
Baumann HJ, Kluge S, Balke L et al (2008) Yield and safety of bedside open lung biopsy in mechanically ventilated patients with acute lung injury or acute respiratory distress syndrome. Surgery 143:426–433
Kao KC, Tsai YH, Wu YK et al (2006) Open lung biopsy in early-stage acute respiratory distress syndrome. Crit Care 10:R106
Charbonney E, Robert J, Pache JC, Chevrolet JC, Eggimann P (2009) Impact of bedside open lung biopsies on the management of mechanically ventilated immunocompromised patients with acute respiratory distress syndrome of unknown etiology. J Crit Care 24:122–128
Parambil JG, Myers JL, Aubry MC, Ryu JH (2007) Causes and prognosis of diffuse alveolar damage diagnosed on surgical lung biopsy. Chest 132:50–57
De Hemptinne Q, Remmelink M, Brimioulle S, Salmon I, Vincent JL (2009) ARDS a clinicopathological confrontation. Chest 135:944–949
Thille AW, Esteban A, Fernandez-Segoviano P et al (2013) Chronology of histological lesions in acute respiratory distress syndrome with diffuse alveolar damage: a prospective cohort study of clinical autopsies. Lancet Respir Med 1:395–401
Thompson BT, Matthay MA (2013) The Berlin definition of ARDS versus pathological evidence of diffuse alveolar damage. Am J Respir Crit Care Med 187:675–677
Acknowledgments
We are indebted to Sylvaine Sazio and Réjane Fievez, the medical secretaries at the Service de Réanimation Médicale et d’Assistance Respiratoire, Lyon, France, for their invaluable help in managing the patient charts for the purposes of the present study and to Prof. Alain Mercat, Angers, France, for his reappraisal of the article.
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Take-home message:
Diffuse alveolar damage is present in the majority of patients with nonresolving ARDs and its frequency is no different across the three ARDS stages. On this basis, the systematic use of steroids in nonresolving ARDS is not recommended.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guerin, C., Bayle, F., Leray, V. et al. Open lung biopsy in nonresolving ARDS frequently identifies diffuse alveolar damage regardless of the severity stage and may have implications for patient management. Intensive Care Med 41, 222–230 (2015). https://doi.org/10.1007/s00134-014-3583-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-014-3583-2