Abstract
Purpose
A new pathway of three protein tyrosine kinase receptors, namely, the TAM receptor family [Tyro-3, Axl and Mer tyrosine kinase (MerTK)], has recently been described to negatively control immune responses. The objective of this prospective, observational, clinical study was to investigate the expression patterns of TAM receptors in circulating white blood cells collected from patients with septic shock.
Methods
The expression of TAM receptors was measured by flow cytometry in circulating leukocytes from patients with septic shock sampled on days (D) 1–2 (n = 47) and D3–4 (n = 37) after the onset of shock, severe trauma patients at D1–2 after trauma (n = 51) and healthy individuals (n = 23).
Results
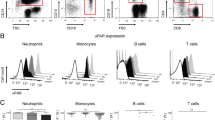
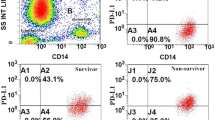
On D1–2 after injury, MerTK was overexpressed in monocytes and neutrophils collected from patients with septic shock in comparison with those collected from healthy volunteers and trauma patients. This phenomenon was also observed for mRNA. Conversely, the expression of Tyro-3 and Axl was higher in monocytes from trauma patients versus healthy volunteers or those in septic shock. MerTK expression between D1–2 and D3–4 remained elevated in patients suffering from septic shock who died or developed an intensive care unit-acquired infection, whereas it decreased in patients who recovered uneventfully. This in vivo observed expression pattern was reproduced ex vivo after the incubation of healthy volunteer cells with plasma from septic shock or trauma patients.
Conclusions
MerTK expression in circulating innate immune cells is increased in patients with septic shock in comparison with healthy volunteers and trauma patients. Persistent MerTK overexpression after septic shock is associated with adverse outcome. The role of this family of receptors in the pathophysiology of injury-induced immune dysfunctions deserves to be specifically investigated.




Similar content being viewed by others
References
Hotchkiss RS, Karl IE (2003) The pathophysiology and treatment of sepsis. N Engl J Med 348:138–150
Levy MM, Artigas A, Phillips GS, Rhodes A, Beale R, Osborn T, Vincent JL, Townsend S, Lemeshow S, Dellinger RP (2012) Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis 12:919–924
Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA (2009) The sepsis seesaw: tilting toward immunosuppression. Nat Med 15:496–497
Monneret G, Venet F, Pachot A, Lepape A (2008) Monitoring immune dysfunctions in the septic patient: a new skin for the old ceremony. Mol Med 14:64–78
Hotchkiss RS, Monneret G, Payen D (2013) Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis 13:260–268
Hafizi S, Dahlback B (2006) Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. FEBS J 273:5231–5244
Benzakour O, Gely A, Lara R, Coronas V (2007) Gas-6 and protein S: vitamin K-dependent factors and ligands for the TAM tyrosine kinase receptors family. Med Sci (Paris) 23:826–833
Lemke G, Rothlin CV (2008) Immunobiology of the TAM receptors. Nat Rev Immunol 8:327–336
Lu Q, Lemke G (2001) Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science 293:306–311
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101:1644–1655
Landelle C, Lepape A, Francais A, Tognet E, Thizy H, Voirin N, Timsit JF, Monneret G, Vanhems P (2008) Nosocomial infection after septic shock among intensive care unit patients. Infect Control Hosp Epidemiol 29:1054–1065
Monneret G, Lepape A, Voirin N, Bohe J, Venet F, Debard AL, Thizy H, Bienvenu J, Gueyffier F, Vanhems P (2006) Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med 32:1175–1183
Venet F, Chung CS, Kherouf H, Geeraert A, Malcus C, Poitevin F, Bohe J, Lepape A, Ayala A, Monneret G (2009) Increased circulating regulatory T cells (CD4(+)CD25 (+)CD127 (-)) contribute to lymphocyte anergy in septic shock patients. Intensive Care Med 35:678–686
Hafizi S, Dahlback B (2006) Signalling and functional diversity within the Axl subfamily of receptor tyrosine kinases. Cytokine Growth Factor Rev 17:295–304
Castoldi E, Hackeng TM (2008) Regulation of coagulation by protein S. Curr Opin Hematol 15:529–536
Rigby AC, Grant MA (2004) Protein S: a conduit between anticoagulation and inflammation. Crit Care Med 32:S336–S341
Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G (2007) TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 131:1124–1136
Lee YJ, Han JY, Byun J, Park HJ, Park EM, Chong YH, Cho MS, Kang JL (2012) Inhibiting Mer receptor tyrosine kinase suppresses STAT1, SOCS1/3, and NF-kappaB activation and enhances inflammatory responses in lipopolysaccharide-induced acute lung injury. J Leukoc Biol 91:921–932
Camenisch TD, Koller BH, Earp HS, Matsushima GK (1999) A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. J Immunol 162:3498–3503
Choi JY, Park HJ, Lee YJ, Byun J, Youn YS, Choi JH, Woo SY, Kang JL (2013) Upregulation of Mer receptor tyrosine kinase signaling attenuated LPS-induced lung inflammation. J Pharmacol Exp Ther 344(2):447–458
O’Neill LA (2007) TAMpering with toll-like receptor signaling. Cell 131:1039–1041
Borgel D, Clauser S, Bornstain C, Bieche I, Bissery A, Remones V, Fagon JY, Aiach M, Diehl JL (2006) Elevated growth-arrest-specific protein 6 plasma levels in patients with severe sepsis. Crit Care Med 34:219–222
Ekman C, Linder A, Akesson P, Dahlback B (2010) Plasma concentrations of Gas6 (growth arrest specific protein 6) and its soluble tyrosine kinase receptor sAxl in sepsis and systemic inflammatory response syndromes. Crit Care 14:R158
Borgel D, Bornstain C, Reitsma PH, Lerolle N, Gandrille S, Dali-Ali F, Esmon CT, Fagon JY, Aiach M, Diehl JL (2007) A comparative study of the protein C pathway in septic and nonseptic patients with organ failure. Am J Respir Crit Care Med 176:878–885
Xu PB, Lou JS, Ren Y, Miao CH, Deng XM (2012) Gene expression profiling reveals the defining features of monocytes from septic patients with compensatory anti-inflammatory response syndrome. J Infect 65(5):380–391
Deng T, Zhang Y, Chen Q, Yan K, Han D (2012) Toll-like receptor-mediated inhibition of Gas6 and ProS expression facilitates inflammatory cytokine production in mouse macrophages. Immunology 135:40–50
Lemmon MA, Schlessinger J (2010) Cell signaling by receptor tyrosine kinases. Cell 141:1117–1134
Sasaki T, Knyazev PG, Clout NJ, Cheburkin Y, Gohring W, Ullrich A, Timpl R, Hohenester E (2006) Structural basis for Gas6-Axl signalling. EMBO J 25:80–87
Brown JE, Krodel M, Pazos M, Lai C, Prieto AL (2012) Cross-phosphorylation, signaling and proliferative functions of the Tyro3 and Axl receptors in Rat2 cells. PLoS One 7:e36800
Gouel-Cheron A, Allaouchiche B, Guignant C, Davin F, Floccard B, Monneret G (2012) Early interleukin-6 and slope of monocyte human leukocyte antigen-DR: a powerful association to predict the development of sepsis after major trauma. PLoS One 7:e33095
Lukaszewicz AC, Grienay M, Resche-Rigon M, Pirracchio R, Faivre V, Boval B, Payen D (2009) Monocytic HLA-DR expression in intensive care patients: interest for prognosis and secondary infection prediction. Crit Care Med 37:2746–2752
Acknowledgments
The authors would like to thank H. Vallin, M. Provent, N. Panel and C. Jouvene-Faure from Lyon-Sud and HEH ICUs (Hospices Civils de Lyon) for collecting patients’ inclusion and clinical data gathering as well as Anne Portier (Hospices Civils de Lyon, Immunology Lab, E. Herriot hospital) for her technical assistance. This work was supported by funds from the Hospices Civils de Lyon (GM).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
C. Guignant and F. Venet contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guignant, C., Venet, F., Planel, S. et al. Increased MerTK expression in circulating innate immune cells of patients with septic shock. Intensive Care Med 39, 1556–1564 (2013). https://doi.org/10.1007/s00134-013-3006-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-013-3006-9