Abstract
Purpose
To compare automated administration of propofol and remifentanil guided by the Bispectral index (BIS) versus manual administration of short-acting drugs in critical care patients requiring deep sedation. The primary outcome was the percentage of BIS values between 40 and 60 (BIS40–60).
Methods
This randomized controlled phase II trial in the intensive care unit (ICU) was conducted in adults with multiorgan failure. Thirty-one patients were assigned to receive sedation with propofol or remifentanil either by an automated or a manual system, both targeting BIS40–60. Performance and feasibility of an automated administration were assessed.
Results
The study groups were well balanced in terms of demographic characteristics. Study duration averaged 18 [8–24] h in the automated group and 14 [9–21] h in the manual group (p = 0.81). Adequate sedation (BIS40–60) was significantly more frequent in the automated group 77 [59–82] % than in the manual group 36 [22–56] %, with p = 0.001. Propofol consumption was reduced by a factor of 2 in the automated group with a median change of infusion rates of 39 ± 9 times per hour. In contrast, there were only 2 ± 1 propofol and 1 ± 1 remifentanil dose changes per hour in the manual group compared to 40 ± 9 for remifentanil in the automated group (p < 0.001). Vasopressors were more often discontinued or reduced in the automated group than in the manual control group (36 [6–40] vs. 12 [4–20] modifications, p = 0.03).
Conclusions
Continuous titration of propofol and remifentanil sedation with an automatic controller maintains deep sedation better than manual control in severely ill patients. It is associated with reduced sedative and vasopressor use.
Similar content being viewed by others
Introduction
Mechanically ventilated patients in intensive care units (ICU) require sedation to improve comfort, reduce psychological stress, control pain, and facilitate nursing procedures [1]. Excessive sedation increases the risk of ventilator-associated pneumonia and subsequent withdrawal syndrome, whereas it prolongs mechanical ventilation and ICU length-of-stay [2, 3]. Careful control of sedation and frequent assessment of individual sedation needs have thus been recommended [4, 5]. Others advocate a daily interruption of sedative infusions [6] or even completely avoiding sedation for mechanically ventilated patients [7].
The amount of sedation required in ICU patients depends on the underlying illness and comorbidities. Sedation requirements also change over time, with sudden nociceptive stimuli in hemodynamically unstable patients making the ICU a challenging environment for sedation. Moreover, physicians and nurses in critical care units have many responsibilities besides sedation and can thus devote only limited time to this task.
Automated sedation has the potential to improve patient care by adjusting drug doses to the minimum required for efficacy. Continuous titration avoids overdosing and consequent hemodynamic side effects which in turn might prompt fluid loading or vasopressor administration; appropriate titration also prevents drug accumulation which facilitates neurological assessments. Automated sedation has been facilitated by the introduction of the Bispectral index (BIS) which permits continuous monitoring of electrocortical activity. Randomized trials show that automated controllers outperform manual intravenous administration for maintaining adequate depth of hypnosis (BIS40–60) during general anesthesia [8]. Automated control also decreases the mean time to tracheal extubation, improves hemodynamic stability, and has been used in patients with severe comorbidities [9, 10]. Recently we demonstrated that a dual closed-loop controller for propofol and remifentanil administration outperforms manual titration during general anesthesia [11].
The aim of this phase II trial was to compare the percentage of adequate sedation, defined as the percentage of time with BIS between 40 and 60 (BIS40–60), with automated or manual administration of propofol–remifentanil in critically ill patients. Specifically, we tested the primary hypothesis that critical care patients are better sedated with automatic BIS-guided dual-loop control than manual control. Secondary hypotheses were that dual-loop control reduces sedative and vasopressor use.
Materials and methods
This prospective randomized single-blind clinical trial was conducted with the approval of the Ethics Committee (Comité de Protection des Personnes (CPP), Boulogne Billancourt, France N°060658) and the relevant French regulatory office. The trial registration number is NCT00393003.
The study was coordinated by Hospital Foch (Suresnes, France) and conducted in the multidisciplinary intensive care unit of the University Hospital Pitié-Salpêtrière (Assistance Publique des Hôpitaux de Paris, Paris, France). Patients recovering from aortic or emergent abdominal surgery and mechanically ventilated for postoperative multiorgan failure were eligible if deep sedation was required for medical reasons (adaptation to mechanical ventilator, reduction of cardiac output, etc.) with a minimal period of one night after admission. The decision to withdraw sedation was left to the attending physician’s discretion, but the propofol infusion did not exceed 48 h to avoid propofol infusion syndrome [12]. We excluded patients less than 18 years old, pregnant women, patients equipped with a pacemaker, patients with a preexistent neurological disease (Parkinson’s disease etc.), and patients whose life expectancy was less than 48 h.
Protocol
Eligibility criteria were determined after surgery upon ICU admission. Written consent was exclusively obtained from each patient’s relatives and acceptation of participation was then confirmed by patients if alive. Patients were randomly assigned to receive either manual (manual group) or automated (automated group) sedation in a 1:1 ratio. The random treatment sequence was computer-generated in blocks of ten; assignments were kept in sequentially numbered sealed opaque envelopes that were opened shortly before treatment began.
In both groups, propofol (Diprivan®, 1 %—AstraZeneca, Rueil-Malmaison, France) and remifentanil (Ultiva®, GSK, Marly-le-roi, France) were infused via a dedicated port of a central venous catheter using the Infusion ToolBox software (ITB 95, version 11.18) [13] with two Asena GH® infusion pumps (Alaris Medical UK Ltd., Basingstoke, Hampshire, UK). Administration of neuromuscular blocking agent (NMBA) and vasopressors was left to the practitioner’s discretion and nurses’ control in accordance with a local protocol.
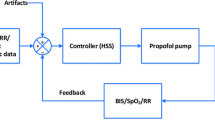
In patients assigned to the manual control group, nurses and physicians followed a local algorithm dedicated to achieve BIS40–60 as a main objective using target-controlled infusion mode with the usual pharmacokinetic model of Schnider et al. [14] and Minto et al. [15] for propofol and remifentanil, respectively (Fig. 1). BIS values (version 3.11; Covidien, Dublin, Ireland) and clinical judgment were the two determinants for the sedation decision in the manual group. Nurses were trained at the bedside prior to the start of the study with theoretical and practical training with medical (MLG) supervision to ensure that decisions were made in accordance with the algorithm. As clinical determinant, the behavioral pain scale described to assess pain in critically ill ventilated patients was used with a threshold to treat above a score of 4 [16] [Appendix 1 (electronic supplementary material, ESM)]. In patients assigned to automated control, BIS40–60 was maintained by a dual closed-loop controller [11], which was previously described in detail. It is based on a proportional integral derivative (PID) algorithm and uses the BIS to calculate the error or the difference between the set point and the measured BIS. If the BIS error is different from 0, the controller determines a new propofol and/or remifentanil concentration. The error size determines which drug will be modified: only remifentanil is changed if the error is small; both drug concentrations are changed if the error is high. Clinicians can override the control system either temporarily or permanently. The main difference in the current algorithm for ICU was the per-default minimal value of the calculated effect-site concentration.
Owing to safety reasons and in accordance with CPP approval, a prolonged sedation with propofol was not performed to avoid risk of propofol-induced syndrome. After the study, sedation was pursued according to the local protocol with midazolam.
Measurements
Demographic characteristics, critical care score [index of gravity scale II (IGSII) [17], acute physiology and chronic health evaluation II (APACHE II] [18], and sequential organ failure assessment (SOFA) [19], surgical procedures, need for emergent surgery, NMBA use, and 28-day mortality were recorded.
In addition to routine and patient-specific care, Ramsay scores were recorded every 3 h before nursing [20]. In parallel to clinical assessment, a BIS value was also recorded. BIS, burst suppression ratio (the fraction of electroencephalogram isoelectric activity detected by the BIS monitor), calculated effect-site concentration, and consumption and number of target changes of propofol and remifentanil were recorded [13]. We defined the suppression ratio (SR) as one episode of an SR value greater than 10 % lasting more than 1 min [21] and the normalized duration of SR was calculated in patients who experienced an SR as the percentage of time with SR during sedation. Use of vasoactive drugs and changes were recorded.
Performance of the system for maintenance of sedation was determined with the following criteria: BIS40–60, percentage of deep (BIS<40), or light sedation (BIS>60). Adverse events were recorded.
Statistical analysis
Our a priori sample-size estimate was based on 80 % power for a 50 % improvement in the fraction of adequate sedation at an alpha risk of 5 %. Thirteen patients per group were necessary, but anticipating that some critically ill patients would be unable to complete the study, we planned to enroll 15 patients per group [22].
Demographic results are reported as medians and interquartile ranges [IQR] or number (percentage). Median and IQR were calculated by bootstrap analysis. A correlation between BIS values and Ramsay score was performed. Automated and manual groups were compared with non-parametric tests (Wilcoxon or Mann–Whitney U tests, as appropriate). P < 0.05 was considered as statistically significant. Statistical analysis was performed with IBM-SPSS® 20.0 (Inc., USA) for Windows.
Results
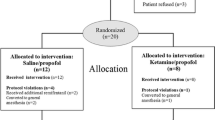
Among 40 qualifying patients or relatives who were approached, 31 patients consented and were randomized. However, one patient in each group was excluded from analysis because electronic data acquisition failed. Analysis was thus restricted to 14 patients in the manual group and 15 in the automatic group (Fig. 2).
The study group was well balanced in terms of demographic and morphometric characteristics, types of surgery, and sedation duration. However, patients assigned to the automated group had a higher IGS II (Table 1). The total length of sedation under the protocol was similar in each group at 18 [8–24] h in patients assigned to automated control and 14 [9–21] h in those assigned to manual control (p = 0.81, Table 1). The proportion of patients requiring vasopressors was similar in both groups at entry (Table 1). However vasopressors were more often discontinued or decreased in the automated than in the manual control group (36 [6–40] vs. 12 [4–20] modifications, p = 0.03).
The propofol and remifentanil infusions were modified much more often under automatic than under manual control (Table 2, Fig. 3). Adequate sedation (BIS40–60) was about twice as common in the automated group at 77 [59–82] % than in the manual group 36 [22–56] % (p = 0.001, Table 2) with no significant difference in clinical assessment. Median propofol consumption was a factor of 2 less with the dual closed-loop controller (p = 0.012, Table 3); in contrast, remifentanil consumption was similar in each group (Table 3). More than 90 % of the patients in each group experienced at least one episode of burst suppression, and the normalized duration of SR was also similar. A weak correlation between BIS values and Ramsay score was demonstrated with R = 0.524, but considering intervals of BIS values (<40, 40–60, and >60) clinical assessment was unable to discriminate deep level of sedation (Ramsay score >4) (Fig. 4).
Bispectral index (BIS) values and calculated effect-site concentrations of propofol (CePropofol) and remifentanil (CeRemifentanil) during the 12 h of continuous recording according to manual or automated administration. Data are given (a) as individual values with a moving average filter of 2-min duration for graphical representation at the left side of each group, and (b) as median values (dark gray line) with 25th and 75th percentile (light gray line) at the right side of each group. The former representation gives an overall view of performance of automated and manual administration (periods of BIS out of range); the latter representation partially reduces these events and are in accordance with Tables 2 and 3
Box-plot representation of Ramsay score related to depth of sedation measured by BIS monitor (very deep sedation with BIS < 40, deep sedation for BIS 40–60, light with BIS > 60). A weak correlation was demonstrated (R = 0.524), but no significant difference in Ramsay score discriminated BIS values. Top of the boxes are the 25th and 75th percentiles, the band near the middle of the box is the 50th percentile (median). The ends of the whiskers represent the 10th and 90th percentiles
No major undesirable events were observed during the study period (Table 3). As might be expected from the baseline illness severity of our patients, 28-day mortality was high but similar in both groups (p = 0.39, Table 3).
Discussion
This randomized clinical trial performed in ICU patients shows three original findings: (1) adequate sedation assessed using BIS was significantly more frequent in the automated group than in the manual group; (2) propofol consumption was reduced by a factor of two in the automated group; (3) vasopressors were more often discontinued or reduced in the automated than in the manual control group.
Clinical or multidimensional sedation scales are currently recommended to titrate sedation in ICU [23]. But these scoring systems provide only an intermittent, discrete, and subjective estimate of sedation and remain inadequate in case of patients receiving NMBA, or high doses of barbiturate. Automated systems were developed for hypnotic, opioid, and NMBA administration depending on directly measurable effects [8]. BIS is a validated continuous measure of hypnotic depth and is widely used to deliver anesthetic drugs [24, 25]. Moreover, BIS is also used to guide sedation in ICU patients [26, 27]. We thus chose BIS as our primary measure of sedative adequacy. There is, though, poor agreement between BIS and subjective sedation scales to discriminate deep sedation (Fig. 4) [28, 29]. This is mainly due to a discrepancy between situations measured by each tool. Multidimensional and behavioral scales better explore arousal state than sedation per se [20]. Conversely BIS assesses the deep sedation level (0 < BIS < 60) more accurately than the Ramsay scale which attributes only a value of 5 or 6 [30]. In this situation BIS40–60 seems to be a common surrogate measurement of deep sedation in ICU [31] and is the one we especially targeted. Situations such as spontaneous respiratory movements and ventilator asynchrony did not signify less accurate BIS monitoring because there is a direct relation between BIS variation and patients’ discomfort [32]. Therefore, noxious stimuli can lead to electrocortical activation and autonomic response if analgesia is not sufficient [11]. The BIS40–60 set point also has the further advantage of decreasing the impact of muscular activity on BIS [33] and improves agreement between BIS values and sedation scales [34, 35]. Indeed, there is a potential “gray zone” of positive bias around BIS 60 that electromuscular artifacts from the forehead mainly explain. Electromuscular activity spreads from 30 to 300 Hz then electrocortical activity from 0.5 to 47 Hz. The overlaps above 30 Hz can contaminate BIS value determination, but deepened sedation shifts the spectral frequencies of electrocortical activity to the left with an overall slowing down that decreases muscular contamination. The influence of neuromuscular blockade varies with depth of sedation. During deep anesthesia (BIS < 45), a bolus of mivacurium produced a minor effect on the BIS value [33]. Conversely, during a light anesthesia (BIS > 65) a bolus of atracurium decreased the BIS [33]. In two cases, muscular artifacts specifically required muscular blockade. In the first case, clinically important shivering (automated group) altered the BIS recording (values around 70 with a high detected EMG) with a complete resolution after NMBA administration. The second case (manual group) also presented a high BIS value related to an infraclinic convulsive state requiring barbiturates.
Automated sedation in the postoperative period was recently reported in patients scheduled for elective cardiac surgery. This single closed-loop controller of propofol provided adequate sedation with a BIS40–60 value of 69 ± 16 %, during 279 ± 171 min, and propofol consumption was 1.2 ± 0.7 mg kg−1 h−1. Moreover, automated sedation decreased the delay of extubation [36]. However in the current study, patients were older, had multiorgan failure, were sedated for a long time, and analgesia was administered automatically. We assume that the tight control of analgesia was also an important sedation factor. Overall, use of a dual-loop controller guided by BIS doubles adequate sedation (BIS40–60) and consequently too deep or light sedation decreases (Table 2) with a continuous titration. Such an incidence of modifications with a preferentially analgesic balance was really not expected because the environment in the ICU seemed less stressful or painful than during surgery. In comparison the occurrence of changes in the manual group was low (>1/h) but has proved nurses’ implication in this sedation protocol with a scheduled visit every 3 h. While it remains possible that automated control would have been nearly as good with fewer automated changes, our results suggest that many more changes are needed in the manual group than would ever be practical for clinicians.
Our controller rapidly reacts by administrating remifentanil and spares propofol administration, thereby avoiding adverse hemodynamic effects [11, 32]. In this way, current studies suggest that light sedation improves patients’ care [6, 37]. Moreover, median propofol consumption was significantly below the maximal infusion rate to avoid propofol infusion syndrome [38]. This relatively low infusion rate improves hemodynamic stability [12, 38] and the vasopressors were three times more likely to be discontinued or have a dose reduction with automated rather than manual control. Another hypothesis is that an automated controller decreases the workload to control sedation and gives more time for hemodynamic optimization.
Retrospective studies reported a direct relationship between the occurrence of burst suppression and morbidity in patients following cardiac arrest [39] or anoxic lesions [40] with a twofold increase in 6-month mortality [41]. But interestingly, lower propofol infusion rates and generally higher BIS values were not paired with a reduction in burst suppression or SR [39]. The high occurrence of SR in the current study is probably related to the definition of SR [21] with a continuous recording every 5 s. But this may suggest a preexistent cerebral failure or an increased susceptibility to anesthetic effects in a context of multiorgan dysfunction with a major risk of hypnotic overdosing whatever the modalities of administration especially during cardiopulmonary bypass [42].
A limitation of our trial is that randomization to automated or manual control was restricted to less than 48 h in a limited number of patients. It is unlikely that such a brief control period would influence the time to tracheal extubation, neuromyopathy, post sedation cognitive dysfunction, or sedative withdrawal syndrome. Furthermore, our study was not powered for these outcomes.
In summary, use of our dual-loop automated controller improved sedation quality in severely ill patients under deep sedation. Furthermore, BIS-controlled sedation reduced propofol consumption by a factor of 2, while simultaneously improving hemodynamic stability. Automated control thus may be preferable to manual control in critically ill patients. Randomized multicenter studies are required to confirm such benefit and assess its impact on patient outcomes.
References
Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET, Chalfin DB, Masica MF, Bjerke HS, Coplin WM, Crippen DW, Fuchs BD, Kelleher RM, Marik PE, Nasraway SA Jr, Murray MJ, Peruzzi WT, Lumb PD (2002) Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med 30:119–141
Soliman HM, Melot C, Vincent JL (2001) Sedative and analgesic practice in the intensive care unit: the results of a European survey. Br J Anaesth 87:186–192
Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G (1998) The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest 114:541–548
Payen JF, Chanques G, Mantz J, Hercule C, Auriant I, Leguillou JL, Binhas M, Genty C, Rolland C, Bosson JL (2007) Current practices in sedation and analgesia for mechanically ventilated critically ill patients: a prospective multicenter patient-based study. Anesthesiology 106:687–695 (quiz 891–682)
Society of Critical Care Medicine and American Society of Health-System Pharmacists (2002) Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Am J Health Syst Pharm 59:150–178
Kress JP, Pohlman AS, O’Connor MF, Hall JB (2000) Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 342:1471–1477
Strom T, Martinussen T, Toft P (2010) A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet 375:475–480
Rinehart J, Liu N, Alexander B, Cannesson M (2012) Review article: closed-loop systems in anesthesia: is there a potential for closed-loop fluid management and hemodynamic optimization? Anesth Analg 114:130–143
Agarwal J, Puri GD, Mathew PJ (2009) Comparison of closed loop vs. manual administration of propofol using the Bispectral index in cardiac surgery. Acta Anaesthesiol Scand 53:390–397
Liu N, Chazot T, Trillat B, Michel-Cherqui M, Marandon JY, Law-Koune JD, Rives B, Fischler M (2008) Closed-loop control of consciousness during lung transplantation: an observational study. J Cardiothorac Vasc Anesth 22:611–615
Liu N, Chazot T, Hamada S, Landais A, Boichut N, Dussaussoy C, Trillat B, Beydon L, Samain E, Sessler DI, Fischler M (2011) Closed-loop coadministration of propofol and remifentanil guided by bispectral index: a randomized multicenter study. Anesth Analg 112:546–557
Kam PC, Cardone D (2007) Propofol infusion syndrome. Anaesthesia 62:690–701
Cantraine FR, Coussaert EJ (2000) The first object oriented monitor for intravenous anesthesia. J Clin Monit Comput 16:3–10
Schnider TW, Minto CF, Gambus PL, Andresen C, Goodale DB, Shafer SL, Youngs EJ (1998) The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology 88:1170–1182
Minto CF, Schnider TW, Egan TD, Youngs E, Lemmens HJ, Gambus PL, Billard V, Hoke JF, Moore KH, Hermann DJ, Muir KT, Mandema JW, Shafer SL (1997) Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil. I. Model development. Anesthesiology 86:10–23
Payen JF, Bru O, Bosson JL, Lagrasta A, Novel E, Deschaux I, Lavagne P, Jacquot C (2001) Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med 29:2258–2263
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Le Gall JR, Neumann A, Hemery F, Bleriot JP, Fulgencio JP, Garrigues B, Gouzes C, Lepage E, Moine P, Villers D (2005) Mortality prediction using SAPS II: an update for French intensive care units. Crit Care 9:R645–R652
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
Ramsay MA (2000) Measuring level of sedation in the intensive care unit. JAMA 284:441–442
Besch G, Liu N, Samain E, Pericard C, Boichut N, Mercier M, Chazot T, Pili-Floury S (2011) Occurrence of and risk factors for electroencephalogram burst suppression during propofol-remifentanil anaesthesia. Br J Anaesth 107:749–756
Liu N, Chazot T, Trillat B, Pirracchio R, Law-Koune JD, Barvais L, Fischler M (2006) Feasibility of closed-loop titration of propofol guided by the Bispectral Index for general anaesthesia induction: a prospective randomized study. Eur J Anaesthesiol 23:465–469
Ramsay MA, Savege TM, Simpson BR, Goodwin R (1974) Controlled sedation with alphaxalone-alphadolone. Br Med J 2:656–659
Rampil IJ (1998) A primer for EEG signal processing in anesthesia. Anesthesiology 89:980–1002
Johansen JW (2006) Update on bispectral index monitoring. Best Pract Res Clin Anaesthesiol 20:81–99
Karamchandani K, Rewari V, Trikha A, Batra RK (2010) Bispectral index correlates well with Richmond agitation sedation scale in mechanically ventilated critically ill patients. J Anesth 24:394–398
Trouiller P, Fangio P, Paugam-Burtz C, Appere-de-Vecchi C, Merckx P, Louvet N, Pease S, Outin H, Mantz J, De Jonghe B (2009) Frequency and clinical impact of preserved bispectral index activity during deep sedation in mechanically ventilated ICU patients. Intensive Care Med 35:2096–2104
Olson DM, Thoyre SM, Peterson ED, Graffagnino C (2009) A randomized evaluation of bispectral index-augmented sedation assessment in neurological patients. Neurocrit Care 11:20–27
Frenzel D, Greim CA, Sommer C, Bauerle K, Roewer N (2002) Is the bispectral index appropriate for monitoring the sedation level of mechanically ventilated surgical ICU patients? Intensive Care Med 28:178–183
Tonner PH, Weiler N, Paris A, Scholz J (2003) Sedation and analgesia in the intensive care unit. Curr Opin Anaesthesiol 16:113–121
Consales G, Chelazzi C, Rinaldi S, De Gaudio AR (2006) Bispectral Index compared to Ramsay score for sedation monitoring in intensive care units. Minerva Anestesiol 72:329–336
Guignard B, Menigaux C, Dupont X, Fletcher D, Chauvin M (2000) The effect of remifentanil on the bispectral index change and hemodynamic responses after orotracheal intubation. Anesth Analg 90:161–167
Liu N, Chazot T, Huybrechts I, Law-Koune JD, Barvais L, Fischler M (2005) The influence of a muscle relaxant bolus on bispectral and datex-ohmeda entropy values during propofol-remifentanil induced loss of consciousness. Anesth Analg 101:1713–1718
Sackey PV (2008) Frontal EEG for intensive care unit sedation: treating numbers or patients? Crit Care 12:186
Nasraway SS Jr, Wu EC, Kelleher RM, Yasuda CM, Donnelly AM (2002) How reliable is the Bispectral Index in critically ill patients? A prospective, comparative, single-blinded observer study. Crit Care Med 30:1483–1487
Solanki A, Puri GD, Mathew PJ (2010) Bispectral index-controlled postoperative sedation in cardiac surgery patients: a comparative trial between closed loop and manual administration of propofol. Eur J Anaesthesiol 27:708–713
Strom T, Toft P (2011) Time to wake up the patients in the ICU: a crazy idea or common sense? Minerva Anestesiol 77:59–63
Vasile B, Rasulo F, Candiani A, Latronico N (2003) The pathophysiology of propofol infusion syndrome: a simple name for a complex syndrome. Intensive Care Med 29:1417–1425
Seder DB, Fraser GL, Robbins T, Libby L, Riker RR (2010) The bispectral index and suppression ratio are very early predictors of neurological outcome during therapeutic hypothermia after cardiac arrest. Intensive Care Med 36:281–288
Menache CC, Bourgeois BF, Volpe JJ (2002) Prognostic value of neonatal discontinuous EEG. Pediatr Neurol 27:93–101
Watson PL, Shintani AK, Tyson R, Pandharipande PP, Pun BT, Ely EW (2008) Presence of electroencephalogram burst suppression in sedated, critically ill patients is associated with increased mortality. Crit Care Med 36:3171–3177
Monk TG, Weldon BC (2010) Anesthetic depth is a predictor of mortality: it’s time to take the next step. Anesthesiology 112:1070–1072
Acknowledgments
Supported in part by Vaincre la Mucoviscidose (Paris, France), by Covidien (Dublin, Ireland) who loaned the Bispectral monitor, and by Alaris Medical (Hampshire, UK) who loaned the Asena GH® infusion pumps for the study. Hôpital Foch, N. Liu, and T. Chazot are patent holders in France for the gain constants and the control algorithm (N° BFF08P669, Institut National de la Propriété Industrielle, France).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Le Guen, M., Liu, N., Bourgeois, E. et al. Automated sedation outperforms manual administration of propofol and remifentanil in critically ill patients with deep sedation: a randomized phase II trial. Intensive Care Med 39, 454–462 (2013). https://doi.org/10.1007/s00134-012-2762-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2762-2