Abstract
Objective
The Surviving Sepsis Campaign (SSC) developed guidelines and treatment bundles for the administration of steroids in adult septic shock. However, it is not clear how this has affected clinical practice or patient outcome.
Design and setting
The SSC has developed an extensive database to assess the overall effect of its guidelines on clinical practice and patient outcome. This analysis focuses on one particular element of the SSC’s management bundle, namely, the administration of low-dose steroids in adult septic shock. This analysis was conducted on data submitted from January 2005 through March 2010 including 27,836 subjects at 218 sites.
Main results
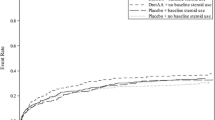
A total of 17,847 (of the total 27,836) patients in the database required vasopressor therapy despite fluid resuscitation and therefore met the eligibility criteria for receiving low-dose steroids. A total of 8,992 patients (50.4 %) received low-dose steroids for their septic shock. Patients in Europe (59.4 %) and South America (51.9 %) were more likely to be prescribed low-dose steroids compared to their counterparts in North America (46.2 %, p < 0.001). The adjusted hospital mortality was significantly higher (OR 1.18, 95 % CI 1.09–1.23, p < 0.001) in patients who received low-dose steroids compared to those who did not. There was still an association with increased adjusted hospital mortality with low-dose steroids even if they were prescribed within 8 h (OR 1.23, 95 % CI 1.13–1.34, p < 0.001).
Conclusions
Steroids were commonly administered in the treatment of septic shock in this subset analysis of the Surviving Sepsis Campaign database. However, this was associated with an increase in adjusted hospital mortality.
Similar content being viewed by others
References
Martin GS, Mannino DM, Eaton S, Moss M (2003) The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348:1546–1554
Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29:1303–1310
Woodward B, Cartwright M (2009) Safety of drotrecogin alfa (activated) in severe sepsis: data from adult clinical trials and observational studies. J Crit Care 24:595–602
Russell JA, Walley KR, Singer J, Gordon AC, Hebert PC, Cooper DJ, Holmes CL, Mehta S, Granton JT, Storms MM, Cook DJ, Presneill JJ, Ayers D (2008) Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 358:877–887
Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, Weiss YG, Benbenishty J, Kalenka A, Forst H, Laterre PF, Reinhart K, Cuthbertson BH, Payen D, Briegel J (2008) Hydrocortisone therapy for patients with septic shock. N Engl J Med 358:111–124
Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D (2006) Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 34:344–353
Maxime V, Lesur O, Annane D (2009) Adrenal insufficiency in septic shock. Clin Chest Med 30:17–27 (vii)
Annane D, Sebille V, Troche G, Raphael JC, Gajdos P, Bellissant E (2000) A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA 283:1038–1045
Annane D, Bellissant E (2000) Prognostic value of cortisol response in septic shock. JAMA 284:308–309
Sprung CL, Annane D, Singer M, Cuthbertson BH, Briegel J (2009) Steroids in patients with septic shock. Chest 136:323–324
LaRosa SP (2005) Use of corticosteroids in the sepsis syndrome: what do we know now? Cleve Clin J Med 72:1121–1127
Lipiner-Friedman D, Sprung CL, Laterre PF, Weiss Y, Goodman SV, Vogeser M, Briegel J, Keh D, Singer M, Moreno R, Bellissant E, Annane D (2007) Adrenal function in sepsis: the retrospective Corticus cohort study. Crit Care Med 35:1012–1018
Groeneveld AB, Molenaar N, Beishuizen B (2008) Should we abandon corticosteroids during septic shock? No. Curr Opin Crit Care 14:384–389
Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, Weiss YG, Benbenishty J, Kalenka A, Forst H, Laterre PF, Reinhart K, Cuthbertson BH, Payen D, Briegel J (2008) Hydrocortisone therapy for patients with septic shock. New Engl J Med 358:111–124
Vincent JL (2008) Steroids in sepsis: another swing of the pendulum in our clinical trials. Crit Care 12:141
Levy MM, Pronovost PJ, Dellinger RP, Townsend S, Resar RK, Clemmer TP, Ramsay G (2004) Sepsis change bundles: converting guidelines into meaningful change in behavior and clinical outcome. Crit Care Med 32:S595–S597
Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM, Ramsay G, Zimmerman JL, Vincent JL, Levy MM (2004) Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 32:858–873
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL (2008) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 36:296–327
Townsend SR, Schorr C, Levy MM, Dellinger RP (2008) Reducing mortality in severe sepsis: the Surviving Sepsis Campaign. Clin Chest Med 29:721–733 (x)
Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, Schorr C, Artigas A, Ramsay G, Beale R, Parker MM, Gerlach H, Reinhart K, Silva E, Harvey M, Regan S, Angus DC, The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 36:222–231
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 31:1250–1256
[cited 2012 May 10] Available from: http://.hhs.gov/ohrp/qualityfaq.html
Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troche G, Chaumet-Riffaud P, Bellissant E (2002) Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 288:862–871
Meduri GU, Belenchia JM, Estes RJ, Wunderink RG, el Torky M, Leeper KV Jr (1991) Fibroproliferative phase of ARDS. Clinical findings and effects of corticosteroids. Chest 100:943–952
Confalonieri M, Urbino R, Potena A, Piattella M, Parigi P, Puccio G, Della Porta R, Giorgio C, Blasi F, Umberger R, Meduri GU (2005) Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med 171:242–248
Beale R, Janes JM, Brunkhorst FM, Dobb G, Levy MM, Martin GS, Ramsay G, Silva E, Sprung CL, Vallet B, Vincent JL, Costigan TM, Leishman AG, Williams MD, Reinhart K (2010) Global utilization of low-dose corticosteroids in severe sepsis and septic shock: a report from the PROGRESS registry. Crit Care 14:R102
Ferrer R, Artigas A, Levy MM, Blanco J, Gonzalez-Diaz G, Garnacho-Montero J, Ibanez J, Palencia E, Quintana M, de la Torre-Prados MV (2008) Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA 299:2294–2303
Kalil AC, Sun J (2008) Why are clinicians not embracing the results from pivotal clinical trials in severe sepsis? A bayesian analysis. PLoS One 3:e2291
Sprung CL, Goodman S, Weiss YG (2009) Steroid therapy of septic shock. Crit Care Clin 25:825–834 (x)
Meduri GU, Belenchia JM, Estes RJ, Wunderink RG, el Torky M, Leeper KV Jr (1991) Fibroproliferative phase of ARDS. Clinical findings and effects of corticosteroids. Chest 100:943–952
Benson K, Hartz AJ (2000) A comparison of observational studies and randomized, controlled trials. N Engl J Med 342:1878–1886
Ferrer R, Artigas A, Suarez D, Palencia E, Levy MM, Arenzana A, Perez XL, Sirvent JM (2009) Effectiveness of treatments for severe sepsis: a prospective, multicenter, observational study. Am J Respir Crit Care Med 180:861–866
Lindenauer PK, Rothberg MB, Nathanson BH, Pekow PS, Steingrub JS (2010) Activated protein C and hospital mortality in septic shock: a propensity-matched analysis. Crit Care Med 38:1101–1107
Acknowledgments
The Surviving Sepsis Campaign was funded in part by unrestricted educational grants from Eli Lilly Co. and Edwards Lifesciences
Conflicts of interest
Brian Casserly, Herwig Gerlach, Gary Philips, John C. Marshall, Stanley Lemeshow and Mitchell M. Levy report no conflicts of interest with regard to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is discussed in the editorial available at: doi:10.1007/s00134-012-2721-y.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Casserly, B., Gerlach, H., Phillips, G.S. et al. Low-dose steroids in adult septic shock: results of the Surviving Sepsis Campaign. Intensive Care Med 38, 1946–1954 (2012). https://doi.org/10.1007/s00134-012-2720-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2720-z