Abstract
Introduction
Physiological dead space should be a routine measurement in ventilated patients but measuring dead space using the Douglas bag (DB) method is cumbersome and requires corrections for compressed ventilator gas. These factors make this method impractical in the critical care setting. Volumetric capnography (VCAP) offers a relatively simple solution to calculating dead space. Few studies have been conducted to directly compare dead space measured by VCAP and the DB method in critically unwell adults.
Method
Prospective observational study of 48 mechanically ventilated adults ICU patients. Dead space was calculated simultaneously using VCAP (CO2SMO) and the Bohr–Enghoff equation. In total, 168 paired readings were taken. Single-breath CO2 waveform areas under the curve were computed automatically by software to calculate physiological dead space. The calculated value of \( P_{{\bar{E}_{{{\text{CO}}_{2} }} }} \) was also recorded from the CO2SMO device. Exhaust ventilator gas was collected in a 10-l mixing chamber. \( P_{{\bar{E}_{{{\text{CO}}_{2} }} }} \) was measured in the chamber following correction for compressed gas.
Results
The study demonstrated good agreement between physiological V D/V T calculated by VCAP and corrected (mean bias 0.03), and uncorrected (mean bias 0.02) Bohr–Enghoff method. There was good correlation between the two methods of measurement (VCAP vs corrected r 2 = 0.90 P < 0.001, VCAP vs uncorrected r 2 = 0.90, P < 0.001). There was good correlation between \( P_{{\bar{E}_{{{\text{CO}}_{2} }} }} \) calculated by the CO2SMO and in the exhaust collected gas (mean bias 0.08).
Conclusions
VCAP shows good agreement with Douglas Bag method in measuring physiological V D/V T over a wide range of dead space fractions.
Similar content being viewed by others
Introduction
Dead space ventilation has been shown consistently to be associated with increased mortality and disease progression in patients with acute lung injury (ALI) acute respiratory distress syndrome (ARDS) [1–5]. Yet in practice, dead space ventilation is seldom used as a clinical tool. This is due largely to the technical problems associated with the traditional methods of measuring dead space.
Calculating dead space ventilation using more traditional methods, such as the Douglas bag (DB), can be impractical and cumbersome. Not least because the equipment required involves a large volume container carried to the bedside. Additionally, the volume of compressed ventilator gas risks over-estimating the dead space fraction [6, 7]. Using volumetric capnography (VCAP) could provide an easier solution to measure dead space and enable clinicians to accrue more data. The aims of this study were to compare physiological dead space using VCAP and the more traditional method of collecting mixed expired gas in a DB.
Methods
The study was approved by the local ethics committee (The National Hospital for Neurology and Neurosurgery and Institute of Neurology Joint REC). Assent was obtained from the family prior to recruitment to the study. The observational study was conducted at the intensive care unit (ICU) at Chelsea and Westminster Hospital—a University hospital in London. All data were collected prospectively between August 2010 and February 2011. Inclusion criteria were age >16 years, invasive positive pressure ventilation, and a PaO2/FiO2 less than 40 kPa at admission. Initial measurements were made following stabilization of the patient and within the first 24 h following admission to the ICU. Measurements were then made once daily until either six consecutive recordings had been made, or the patient had been weaned from the ventilator or died, whichever was the shorter period. For each set of measurements a note was made of the mode of ventilation; mandatory (SIMV or BIPAP) or spontaneous (ASB). All modes of ventilation used non-bias flow triggering with the Drager Evita XL ventilator (Drägerwerk, Lübeck, Germany). Three main variables for analysis were physiological dead space, mixed expired PCO2, and expired tidal volume.
Dead space using modified DB
Dead space fraction was calculated using the Enghoff modification of the Bohr equation, which states:
where \( {\text{Pa}}_{{{\text{CO}}_{2} }} \) is the arterial PCO2 and \( P_{{\bar{E}_{{_{{CO_{2} }} }} }} \) is the mixed expired PCO2. A modified DB was created by attaching a fan to the bottom of a sealed 10-l Perspex chamber. This was used to collect and mix the expired gas from the exhaust port of the ventilator. The carbon dioxide concentration of this expired gas was measured using a BCI Capnocheck capnograph (Smiths Medical, http://www.smiths-medical.com).
One of the drawbacks using the DB method (V D/V T DB) for calculating dead space fraction is that the collected expired gas contains compressed gas from the ventilator. It is difficult to separate this compressed gas from the true expired volume, which leads to diluting and lowering of the measured PCO2 of the expired gas leading to overestimation of V D/V T. Mixed expired CO2 (\( P_{{\bar{E}_{{{\text{CO}}_{2} }} }} \)) was calculated by correcting for gas compression in the ventilator circuit using the method described by Forbat [8]. The corrected tidal volume was calculated using the following equation:
where \( V_{{\text{T}_{C} }} \) is the corrected tidal volume, P B is the barometric pressure, P peak is the peak airway pressure, and V V is the compressible volume of the ventilator and connection tubing. This was set as 1,555 ml for all patients as stated by the manufacturer of the ventilator. The corrected mixed-expired gas was then calculated as follows:
where \( P\bar{E}_{{{\text{CO}}_{2m} }} \) is the measured PCO2 in the mixing chamber. The correction method described has been shown to be comparable to physical separation of expired gas from compressed gas [8]. The mean compressible volume was 2.6 (±0.17) ml/cmH2O, which is comparable to other studies where compressible gas volume has been measured [4].
Following the attachment of the mixing chamber and capnograph to the ventilator expiratory exhaust port, a period of 10 min was allowed to approach steady state, before \( \dot{V}_{\text{E}} \) was recorded, \( P_{{\bar{E}_{{{\text{CO}}_{2} }} }} \) was measured and corrected, and arterial blood drawn for measurement of \( {\text{Pa}}_{{{\text{CO}}_{2} }} \).
Dead space using VCAP
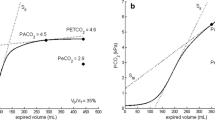
Dead space using VCAP (V D/V T VCAP) was calculated using the CO2SMO®Plus capnograph (Novometrix Medical Systems, Wallingford, CT). The monitor consists of a CO2 sensor that measures CO2 concentrations using infrared absorption technology. A flow sensor is connected to the capnostat and attached proximal to the Y-tubing. The integration of flow with CO2 concentrations allows the monitor to construct a single-breath CO2 (SBT-CO2) waveform. The SBT-CO2 waveform can used to derive dead space and its components as described by Fletcher [9]. The software programme Analysis Plus! for Windows (Novometrix Medical Systems) was used to estimate values for physiological dead space and its two components, anatomical dead space and alveolar dead space, using areas under the SBT-CO2 waveform as described by Fletcher and colleagues [10]. Analysis Plus! automatically computes the areas X, Y, and Z as seen in Fig. 1. Physiological dead space is defined as (Y + Z)/(X + Y + Z).
Components of a single-breath CO2 (SBT-CO2) expirogram as described by Fletcher et al. [10]. Area X represents the CO2 elimination volume, area Y is the alveolar dead space volume, and area Z is the airway dead space volume, where areas p and q are equal. Area Y + Z represents dead space volume. Area (Y + Z)/Area (X + Y + Z) represents dead space fraction
The CO2SMO®Plus monitor estimates \( P_{{\bar{E}_{{{\text{CO}}_{2} }} }} \) by averaging the volumetric CO2 concentration over 1 min. This is calculated by dividing the volume of CO2 over 1 min by the total expired volume over the same period. \( P_{{\bar{E}_{{{\text{CO}}_{2} }} }} \) is the volume weighted eight breath average of this calculation, and is updated every breath. The \( P_{{\bar{E}_{{{\text{CO}}_{2} }} }} \) values estimated by the monitor and the measured and corrected values from the mixing chamber were compared. The expired tidal volume (V Te) as recorded by the ventilator (measure and corrected) were compared with values measured by CO2SMO®Plus monitor. Prior to each reading, both capnographs underwent internal self-calibration.
Statistical analysis
Mean values of continuous normally distributed variables are expressed as mean ± standard deviation. Non-parametric data are presented as median (inter-quartile range). Pearson’s correlation coefficient was used to examine correlation of measured values between measurements. The Bland-Altman method was used to assess agreement between paired measurements of V D/V T, \( P_{{\bar{E}_{{{\text{CO}}_{2} }} }}, \) and V Te [11].
Results
A total of 48 patients (33 men and 15 women) were recruited to the study. Table 1 summarizes the demographic data and admission diagnoses for the population. Table 2 summarizes the baseline physiological variables of the patients at admission. In total, 168 pairs of daily simultaneous readings were taken. Table 3 summarizes the mean values of V D/V T, \( P_{{\bar{E}_{{{\text{CO}}_{2} }} }}, \) and V Te using VCAP, corrected and uncorrected DB method in all readings.
Physiological dead space
Bland-Altman analysis showed good agreement between V D/V T VCAP and uncorrected V D/V T DB, with a mean bias of 0.03 with a 95 % limits of agreement between −0.04 and 0.11 (Fig. 2a). The agreement between V D/V T VCAP and corrected V D/V T DB was similar with a mean bias of 0.02 with 95 % agreement limit between −0.06 and 0.10 (Fig. 2b). There was strong positive correlation between V D/V T VCAP and both uncorrected and corrected V D/V T DB (r = 0.96, r 2 = 0.91; r = 0.95, r 2 = 0.90, respectively; P < 0.001 for both; see Fig. 3).
Bland-Altman plot of differences versus average of paired measured values for physiological dead space. The dotted lines represent the 95 % agreement limit. a Plot of agreement between the volumetric capnography (VCAP) and uncorrected Douglas bag (DB) method. b Plot of agreement between the VCAP method and corrected DB method
Mixed-expired CO2
Bland-Altman analysis of \( P_{{\bar{E}_{{{\text{CO}}_{2} }} }} \) showed good agreement between measurements taken from the CO2SMO monitor and the uncorrected (bias 0.08 kPa, 95 % agreement limit −0.3 to 0.46 kPa; Fig. 4a) and corrected values (bias −0.19 kPa, 95 % agreement limit −0.61 to 0.22 kPa; Fig. 4b) obtained in the mixing chamber. V D/V T was calculated using Eq. 1, where \( P_{{\bar{E}_{{{\text{CO}}_{2} }} }} \) was derived from the CO2SMO and showed good agreement with the DB method (mean bias 0.04, 95 % agreement limit −0.09 to 0.18).
Bland-Altman plot of differences versus average of paired measured values of \( P_{{\bar{E}_{{{\text{CO}}_{2} }} }}.\)The dotted lines represent the 95 % agreement limit. a Plot of agreement between the values derived from VCAP and uncorrected DB method. b Plot of agreement between the values derived from VCAP and corrected DB method
Expired tidal volume
Bland-Altman analysis of uncorrected V Te measured by the ventilator and the CO2SMO monitor revealed a relatively large mean bias of 73.9 ml, with a 95 % agreement limit of −0.8 and 148.5 ml. The agreement between corrected ventilator V Te and the CO2SMO monitor V Te was better, with a mean bias of 43.6 ml and 95 % agreement limit between −90.1 ml and 78.4 ml. All the readings were split into three groups according to the V Te measured by the ventilator: V Te < 400 ml, V Te 400–650 ml, VTe > 650 ml. Bland-Altman analysis of corrected V Te and V Te measured by CO2SMO were as follows: V Te < 400 ml mean bias −25.8 ± 35.7 ml, V Te 400–650 ml mean bias 6.0 ± 42.1 ml, V Te > 650 ml mean bias 21.6 ± 41.9 ml. Linear regression analysis showed V Te CO2SMO = 0.91 × VTe Ventilator − 22.1 (Fig. 5).
Discussion
The results of this study show that there was good agreement between dead space as measured by the more conventional method of collecting mixed-expired gas and as measured by VCAP using areas under the SBT-CO2 waveform. There was also good agreement between \( P_{{\bar{E}_{{{\text{CO}}_{2} }} }} \) measured by VCAP and corrected and uncorrected values measured in expired collected gas. There was poor agreement between expired tidal volume measured by the ventilator and the CO2SMO monitor. After correction for compressed gas, agreement between the two measured tidal volumes improved, particularly in those patients ventilated with 400–650 ml breaths.
Calculating dead space using a DB is impractical in busy intensive care units. Information regarding dead space ventilation is important because it can be used for both disease progression and prognostication. The findings of this study show that there was reasonable agreement between physiological dead space calculated using CO2SMO plus and its software programme Analysis Plus! and the DB method. The additional correction for compressible gas reduced the mean bias between the two methods only marginally. The mean percentage bias across a wide range of physiological dead space abnormalities was 2.5 % (±7.6 %). These margins are unlikely to represent a clinically significant value
The VCAP method of calculating physiological dead space fraction with the DB method has previously been compared in a lung model and in an animal model [12]. The latter study showed significant correlation between the two methods (r 2 = 0.84, P < 0.001) with a bias of 0.02. The findings of our study show a stronger correlation between the two methods, with a similar bias. The findings of this study were also similar to those of a previous study comparing physiological dead space calculated using VCAP and metabolic cart [13]. In turn the metabolic cart has been compared favourably to the DB method of calculating dead space in the paediatric population [14].
The values of \( P_{{\bar{E}_{{{\text{CO}}_{2} }} }} \) derived by CO2SMO also showed reasonable agreement with the DB method. Calculating V D/V T using this value showed good agreement with the corrected DB method (mean bias 6.7 ± 10.1 %). The relevance of this finding is that current generations of VCAP use this measurement to calculate dead space. Such devices are increasingly being incorporated into standard ventilator monitoring [15].
An unexpected finding of the study showed that the agreement between VCAP derived \( P_{{\bar{E}_{{{\text{CO}}_{2} }} }} \) was better with the uncorrected \( P_{{\bar{E}_{{{\text{CO}}_{2} }} }} \)than corrected \( P_{{\bar{E}_{{{\text{CO}}_{2} }} }}.\) This may be explained in part by the use of the correction factor as described by Forbat and colleagues. In their study the correction factor was validated in a very small group of patients across a relatively small range of minute ventilation. All patients were ventilated using volume controlled ventilation [8]. Patients in this study were ventilated with both volume and pressure controlled ventilation. It would be fair to assume that the dynamics of compressed gas in latter group of patients might behave differently. The agreement between corrected V Te and CO2SMO was much better in the range of 400–650 ml. Either side of these tidal volumes, the mean bias increased. This suggests that correction factor described by Forbat and colleagues is unreliable when the tidal volumes are either small or very large.
CO2 rebreathing has been identified as a source of error in dead space calculation [16]. Another advantage of using VCAP is that inspired PCO2 is factored into the calculated value of \( P_{{\bar{E}_{{{\text{CO}}_{2} }} }}. \) Aside from dead space measurements, VCAP measures flow and calculates tidal volume at the airway opening [17]. Values generated by the capnograph are much more likely to represent true expired volumes in comparison to expired volumes calculated by the pneumotachograph in the ventilator, which has inherent inaccuracies of compressed gas. VCAP also allows for calculation of CO2 elimination (\( \dot{V}{\text{CO}}_{2} \)). Measuring \( \dot{V}{\text{CO}}_{2} \) is essential in the complete understanding the efficiency of CO2 elimination by the lungs.
There are several limitations to this study. Firstly, the data was collected from a convenience sample, i.e., only patients with respiratory failure were included into the study. This was in order to capture a group of patients anticipated to have a wide range ventilation-perfusion mismatch. Secondly, two different measurement systems were used to measure PCO2 in order to derive \( P_{{\bar{E}_{{{\text{CO}}_{2} }} }}.\) The Douglas Bag method used the BCI capnocheck whilst the \( P_{{\bar{E}_{{{\text{CO}}_{2} }} }} \) derived by the CO2SMO used the capnostat. Using two different methods to measure the same variable would subject the readings to inaccuracies and errors individual to each device. Ideally, both measurements would have been made using the capnostat. This was not possible as the CO2 analyser of the capnostat requires a steady waveform to measure PCO2; this was not feasible for the Douglas bag.
In summary, physiological dead space fraction derived using VCAP either using the areas under the SBT-CO2 waveform or from derived \( P_{{\bar{E}_{{{\text{CO}}_{2} }} }} \) shows good agreement with the DB method. Using VCAP offers a simple solution to measuring dead space at the bedside and its use in critically unwell patients is likely to improve our understanding of ventilatory efficiency.
References
Cepkova M, Kapur V, Ren X, Quinn T, Zhuo H, Foster E, Liu KD, Matthay MA (2007) Pulmonary dead space fraction and pulmonary artery systolic pressure as early predictors of clinical outcome in acute lung injury. Chest 132:836–842
Kallet RH, Alonso JA, Pittet JF, Matthay MA (2004) Prognostic value of the pulmonary dead-space fraction during the first 6 days of acute respiratory distress syndrome. Respir Care 49:1008–1014
Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, Matthay MA (2002) Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med 346:1281–1286
Raurich JM, Vilar M, Colomar A, Ibanez J, Ayestaran I, Perez-Barcena J, Llompart-Pou JA (2010) Prognostic value of the pulmonary dead-space fraction during the early and intermediate phases of acute respiratory distress syndrome. Respir Care 55:282–287
Siddiki H, Kojicic M, Li G, Yilmaz M, Thompson TB, Hubmayr RD, Gajic O (2010) Bedside quantification of dead-space fraction using routine clinical data in patients with acute lung injury: secondary analysis of two prospective trials. Crit Care 14:R141
Crossman PF, Bushnell LS, Hedley-Whyte J (1970) Dead space during artificial ventilation: gas compression and mechanical dead space. J Appl Physiol 28:94–97
Forbat AF, Her C (1980) Correction for gas compression in mechanical ventilators. Anesth Analg 59:488–493
Forbat AF, Her C (1980) Correction for gas compression in mechanical ventilators. Anesth Analg 59:488–493
Fletcher R, Jonson B, Cumming G, Brew J (1981) The concept of deadspace with special reference to the single breath test for carbon dioxide. Br J Anaesth 53:77–88
Fletcher R, Jonson B, Cumming G, Brew J (1981) The concept of deadspace with special reference to the single breath test for carbon dioxide. Br J Anaesth 53:77–88
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Arnold JH, Thompson JE, Arnold LW (1996) Single breath CO2 analysis: description and validation of a method. Crit Care Med 24:96–102
Kallet RH, Daniel BM, Garcia O, Matthay MA (2005) Accuracy of physiologic dead space measurements in patients with acute respiratory distress syndrome using volumetric capnography: comparison with the metabolic monitor method. Respir Care 50:462–467
Lum L, Saville A, Venkataraman ST (1998) Accuracy of physiologic deadspace measurement in intubated pediatric patients using a metabolic monitor: comparison with the Douglas bag method. Crit Care Med 26:760–764
Sinha P, Flower O, Soni N (2011) Deadspace ventilation: a waste of breath! Intensive Care Med 37:735–746
Fletcher R, Werner O, Nordstrom L, Jonson B (1983) Sources of error and their correction in the measurement of carbon dioxide elimination using the Siemens-Elema CO2 Analyzer. Br J Anaesth 55:177–185
Castle RA, Dunne CJ, Mok Q, Wade AM, Stocks J (2002) Accuracy of displayed values of tidal volume in the pediatric intensive care unit. Crit Care Med 30:2566–2574
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sinha, P., Soni, N. Comparison of volumetric capnography and mixed expired gas methods to calculate physiological dead space in mechanically ventilated ICU patients. Intensive Care Med 38, 1712–1717 (2012). https://doi.org/10.1007/s00134-012-2670-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2670-5