Abstract
Purpose
To investigate the role of myeloid-derived suppressor cells (MDSCs) during sepsis in mice. MDSCs are a heterogeneous population of cells that expand during cancer, inflammation and infection. These cells, by their ability to suppress T lymphocyte proliferation, regulate immune responses during various diseases. Their role during microbial infections is scarcely known.
Methods
Septic shock was induced by caecal ligation and puncture in adult male BALB/c mice; sham-operated animals served as controls. Animals were killed under anaesthesia to harvest blood and organs.
Results
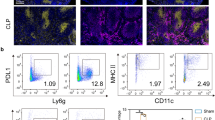
Polymicrobial sepsis induced a progressive accumulation of MDSCs in spleens that were found to be enlarged in surviving mice. MDSCs harvested at day 10 after the onset of infection were highly responsive to LPS in terms of cytokines secretion, NF-kB activation, ROS production and arginase I activity, whereas early-appearing (day 3) MDSCs poorly responded to this stimulus. By contrast, both day 3 and day 10 MDSCs were able to inhibit T cell proliferation. Adoptive transfer of day 10 MDSCs to septic mice attenuated peritoneal cytokine production, increased bacterial clearance and dramatically improved survival rate.
Conclusion
These results provide new information on the role of MDSCs, suggesting a protective effect during sepsis. Pharmacologic agents known to promote the expansion of MDSCs should thus be further studied for sepsis treatment.




Similar content being viewed by others
Abbreviations
- AT:
-
Adoptive transfer
- CLP:
-
Caecal ligation and puncture
- GSK:
-
Glycogen synthase kinase
- IL:
-
Interleukin
- LPS:
-
Lipopolysaccharide
- MDSC:
-
Myeloid-derived suppressor cells
- MO-MDSC:
-
Macrophage-like MDSC
- PMN-MDSC:
-
Neutrophil-like MDSC
- ROS:
-
Reactive oxygen species
- TLR:
-
Toll-like receptor
References
Martin GS, Mannino DM, Eaton S, Moss M (2003) The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348:1546–1554
Hotchkiss RS, Karl IE (2003) The pathophysiology and treatment of sepsis. N Engl J Med 348:138–150
Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9:162–174
Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA (2008) Identification of discrete tumor‐induced myeloid‐derived suppressor cell subpopulations with distinct T cell‐suppressive activity. Blood 111:4233–4244
Youn JI, Nagaraj S, Collazo M, Gabrilovich DI (2008) Subsets of myeloid‐derived suppressor cells in tumor‐bearing mice. J Immunol 181:5791–5802
Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V (2010) Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol 22:238–244
Munera V, Popovic PJ, Bryk J, Pribis J, Caba D, Matta BM, Zenati M, Ochoa JB (2010) Stat-6 dependent induction of myeloid-derived suppressor cells after physical injury regulates nitric oxide response to endotoxin. Ann Surg 251:120–126
Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S (2006) Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol 176:284–290
Noel G, Wang Q, Schwemberger S, Hanson C, Giacalone N, Haar L, Ogle CK (2011) Neutrophils, not monocyte/macrophages, are the major splenic source of postburn IL-10. Shock 36:149–155
Cripps JG, Gorham JD (2011) MDSC in autoimmunity. Int Immunopharmacol 11:789–793
Goni O, Alcaide P, Fresno M (2002) Immunosuppression during acute Trypanosoma cruzi infection: involvement of Ly6G (Gr1(+))CD11b(+)immature myeloid suppressor cells. I. Int Immunol 14:1125–1134
Voisin MB, Buzoni-Gatel D, Bout D, Velge-Roussel F (2004) Both expansion of regulatory GR1 + CD11b + myeloid cells and anergy of T lymphocytes participate in hyporesponsiveness of the lung-associated immune system during acute toxoplasmosis. Infect Immun 72:5487–5492
Cuervo H, Guerrero NA, Carbajosa S, Beschin A, De Baetselier P, Girones N, Fresno M (2011) Myeloid-derived suppressor cells infiltrate the heart in acute Trypanosoma cruzi infection. J Immunol 187:2656–2665
Pereira WF, Ribeiro-Gomes FL, Guillermo LV, Vellozo NS, Montalvao F, Dosreis GA, Lopes MF (2011) Myeloid-derived suppressor cells help protective immunity to Leishmania major infection despite suppressed T cell responses. J Leuk Biol 90:1191–1197
Chen S, Akbar SM, Abe M, Hiasa Y, Onji M (2011) Immunosuppressive functions of hepatic myeloid-derived suppressor cells of normal mice and in a murine model of chronic hepatitis B virus. Clin Exp Immunol 166:134–142
Tacke R, Lee HC, Goh C, Courtney J, Polyak SJ, Rosen HR, Hahn YS (2011) Myeloid suppressor cells induced by hepatitis C virus suppress T cell responses through the production of reactive oxygen species. Hepatology. doi:10.1002/hep.24700
Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O’Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL (2007) MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med 204:1463–1474
Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moreno C, Scumpia PO, Laface DM, Heyworth PG, Efron PA, Moldawer LL (2011) A paradoxical role for myeloid derived suppressor cells in sepsis and trauma. Mol Med 17:281–292
Gibot S, Kolopp-Sarda MN, Béné MC, Bollaert PE, Lozniewski A, Mory F, Levy B, Faure GC (2004) A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J Exp Med 200:1419–1426
Ford JW, McVicar DW (2009) TREM and TREM-like receptors in inflammation and disease. Curr Opin Immunol 21:38–46
Youn JI, Gabrilovich DI (2010) The biology of myeloid-derived suppressor cells, the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol 40:2969–2975
Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S (2007) Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res 67:10019–10026
Sander LE, Sackett SD, Dierssen U, Beraza N, Linke RP, Müller M, Blander JM, Tacke F, Trautwein C (2010) Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J Exp Med 207:1453–1464
Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau D, De Thonel A, Multhoff G, Hamman A, Martin F, Chauffert B, Solary E, Zitvogel L, Garrido C, Ryffel B, Borg C, Apetoh L, Rébé C, Ghiringhelli F (2010) Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest 120:457–471
Yoshimura A, Ohishi HM, Aki D, Hanada T (2004) Regulation of TLR signaling and inflammation by SOCS family proteins. J Leuk Biol 75:422–427
Wang H, Brown J, Martin M (2011) Glycogen synthase kinase 3: a point of convergence for the host inflammatory response. Cytokine 53:130–140
Ostrand-Rosenberg S, Sinha P (2009) Myeloid-derived suppressor cells, linking inflammation and cancer. J Immunol 182:4499–4506
Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB (2006) CD11b +/Gr-1 + myeloid suppressor cells cause T cell dysfunction after traumatic stress. J Immunol 176:2085–2094
Adib-Conquy M, Cavaillon JM (2009) Compensatory anti-inflammatory response syndrome. Thromb Haemost 101:36–47
Noel G, Wang Q, Osterburg A, Schwemberger S, James L, Haar L, Giacalone N, Thomas I, Ogle C (2010) A ribonucleotide reductase inhibitor reverses burn-induced inflammatory defects. Shock 34:535–544
Delano MJ, Thayer T, Gabrilovich S, Kelly-Scumpia KM, Winfield RD, Scumpia PO, Cuenca AG, Warner E, Wallet SM, Wallet MA, O’Malley KA, Ramphal R, Clare-Salzer M, Efron PA, Mathews CE, Moldawer LL (2011) Sepsis induces early alterations in innate immunity that impact mortality to secondary infection. J Immunol 186:195–202
Acknowledgments
We are indebted to Chantal Montemont, Anne-Marie Carpentier and Kevin Patron for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is discussed in the editorial available at: doi:10.1007/s00134-012-2575-3.
Electronic supplementary material
Below is the link to the electronic supplementary material.
134_2012_2574_MOESM1_ESM.doc
Supplementary material 1 (DOC 187 kb) LPS-induced gene expression in D3 and D10 MDSCs. Data are representative of three different experiments, normalized by five housekeeping genes and expressed in 2-ΔCt and fold differences of gene expressions between basal and LPS stimulated (100 ng/mL, 6 h) cells. ND non-detectable
Rights and permissions
About this article
Cite this article
Derive, M., Bouazza, Y., Alauzet, C. et al. Myeloid-derived suppressor cells control microbial sepsis. Intensive Care Med 38, 1040–1049 (2012). https://doi.org/10.1007/s00134-012-2574-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2574-4