Abstract
Purpose
Comparisons of negative versus positive pressure ventilation have imperfectly matched the pressure–time profile or the lung volume history, or have incompletely applied in vivo negative pressure to include the complete thoracic wall and abdomen.
Hypothesis
Negative pressure exerts the same pattern of lung distension as positive pressure when the pressure–time and volume history profiles are identical and the application of negative pressure is over the whole lung.
Methods
(1) In isolated (ex vivo) and (2) intact (in vivo) mouse lungs (n = 4/group) (sealed chamber enclosing either the whole lung or whole mouse except for external airway opening), identical and inverse-tidal, square-wave pressure–time profiles were obtained with positive and negative pressure ventilation. (3) Following an identical volume history, surfactant-depleted rabbits (n = 7) were randomly assigned to sustained, static equivalent positive versus negative pressures. (4) Surfactant-depleted anesthetized rabbits (n = 10) with identical volume histories were randomized to positive versus negative ventilation with identical pressure–time characteristics.
Results
Matched positive and negative pressure time profiles in ex vivo and in vivo mice resulted in identical tidal volumes. Identical (negative vs. positive) sustained static pressures resulted in similar PaO2 and end expiratory lung volumes. Positive and negative ventilation with identical volume histories and pressure time characteristics showed no difference in oxygenation or lung volumes. Historical comparisons suggested better oxygenation with negative pressure when the volume history was not identical.
Conclusions
These data do not support major biological differences between negative and positive pressure ventilation when waveforms and lung volume history are matched.
Similar content being viewed by others
Introduction
Negative pressure ventilation that is applied to the thorax and abdomen achieves lung inflation by distending the rib cage and abdomen. It contrasts with positive pressure ventilation in which lung distension occurs through increasing pressure in the airways. Transpulmonary pressure (P L) is the distending pressure across the lung defined by the pressure at the airway opening (Pao) minus pleural pressure; as such, a drop in overall pleural pressure should have the same mechanical impact as an identical rise in airway opening pressure. However, comparisons of negative and positive pressure ventilation have had mixed results in terms of hemodynamics [1–3], respiratory mechanics [1], oxygenation [4], and on outcome in patients with obstructive lung disease [5].
Recently Grasso and colleagues [6] reported that negative pressure ventilation resulted in better oxygenation that was associated with increased recruitment of atelectatic lung; these findings were supported by comparisons of dynamic positive versus negative pressure inflations. However, unless the transpulmonary pressure was perfectly matched at each instant in both modes of ventilation, differences in lung distension could have resulted from differences in applied transpulmonary pressures as opposed to intrinsic differences between positive versus negative pressure ventilation.
Comparison of static inflation pressure avoids the difficulties associated with matching dynamic pressures because the transpulmonary pressure resulting from positive versus negative pressure would be the same (i.e., at equilibrium); in addition, under steady-state conditions all respiratory movements (i.e., lungs, pleura, chest wall) will have temporarily ceased.
‘Lung volume history’ is known to impact the subsequent mechanical characteristics of the lung [7]; this is incompletely understood, but may involve alterations, such as regional differences in recruitment, surfactant, or tissue matrix, that can exert persistent but non-permanent changes in lung micromechanics and alter the lung response to subsequent inflation pressure.
Application of negative pressure can be either uniform to the chest, abdomen, and limbs (e.g., iron lung [8]) or partial (e.g., selective application to the chest [9] or to the anterior abdominal wall [10]). When applied only to the chest (e.g., using a cuirass negative pressure system) pulmonary blood flow is increased during inflation because of flow from the abdomen into the thorax; however, when applied uniformly to the chest and the abdomen, the distending pressure is (almost) instantaneously distributed across all elements of the respiratory system with no such movement of blood into the thorax.
The current study used ex vivo and in vivo experimental lung models (to account for potential impact of chest wall elastance), with and without surfactant depletion, to compare tidal dynamic inflation with positive or negative pressure. In addition, static inflation was compared after establishing identical lung volume history. In all cases, negative pressure was globally applied, i.e., to the whole lung in ex vivo preparations and to the whole body caudal to the neck in in vivo preparations.
Methods
Institutional ethics approval (conforming to the guidelines of the Canadian Council for Animal Care) was obtained for all experiments. The experimental sequences are outlined in Appendix A (supplementary material).
Isolated mouse lungs
Four male C57/BL6 mice (22–28 g; Charles River Labs, St. Constant, Québec, Canada) were anesthetized with ketamine and xylazine (IP), a tracheostomy tube inserted, and ventilation commenced using an isolated mouse lung apparatus (Type 839; Hugo Sachs Elektronik, March-Hugstetten, Germany) where the lungs were ventilated but not perfused in situ but ex vivo [11]. Ventilation utilized a pressure-controlled ventilator (V T 140–160 μL; PEEP 3 cmH2O; Plugsys Ventilation Control Module-681, Hugo Sachs Elektronik, March-Hugstetten, Germany). The inspired gas comprised 5% CO2, 30% O2, and 65% N2. The chamber pressure was measured with a differential pressure transducer (MPX, Type 399/2; Hugo Sachs Elektronik, March-Hugstetten, Germany). The V T, as well as inspiratory and expiratory flow rates, was measured using a pneumotachograph and a transducer (Validyne DP45, Very Low Range Differential Pressure Transducer; Validyne Engineering Corp., Northridge, CA) previously described under such experimental conditions [11]. Each mouse was ventilated with positive pressure ventilation followed by negative pressure ventilation or vice versa. All lungs were subjected to inspiratory P L of 7.5, 10, and 15 cmH2O (RR 90 per min, FiO2 0.3, end-expiratory P L 3 cmH2O), and V T was measured at each inspiration. Total ventilation time was 10 min.
In vivo mouse, tidal ventilation
Four male C57/BL6 mice were anesthetized, a tracheostomy tube inserted, and ventilation commenced using an isolated mouse lung apparatus (Type 839; Hugo Sachs Elektronik, March-Hugstetten, Germany) in which the mice were ventilated but the chest wall remained intact. Ventilation utilized a pressure-controlled ventilator (V T 140–160 μL; PEEP 3 cmH2O). The inspired gas comprised 5% CO2, 30% O2, and 65% N2. The chamber pressure was measured with a differential pressure transducer (MPX, Type 399/2; Hugo Sachs Elektronik, March-Hugstetten, Germany). The V T and flow rates were measured as above. Each mouse was ventilated with positive pressure ventilation followed by negative pressure ventilation or vice versa. All lungs were subjected to inspiratory P L of 7.5, 10, and 15 cmH2O (RR 90 per min, FiO2 0.3, end-expiratory P L 3 cmH2O), and V T was measured at each inspiration. Total ventilation time was 10 min.
In vivo rabbit, sustained inflation (surfactant-depleted)
Seven New Zealand white rabbits (2.5–3.0 kg; Charles River, QC, Canada) were anesthetized with ketamine and xylazine (IM) and phenobarbital (IV) and a tracheostomy performed [12]. An esophageal catheter was inserted, its position confirmed with the occlusion technique, and baseline (positive pressure) ventilation commenced (V T 9 mL/kg, PEEP 2 cmH2O, RR 37, FiO2 1.0). Surfactant depletion was accomplished with saline lavage, which was repeated until the PaO2 was below 100 mmHg for longer than 30 min.
The rabbit was placed in the iron lung (Portalung Inc. Colorado, USA) that was sealed and enclosed the whole body below the neck. Each animal was randomized to receive either positive or negative pressure ventilation and was then further randomized to static inflation with each of the following P L: 5, 10, 15, or 20 cmH2O (in random order), each for 20 s. At the end of each inflation, an arterial blood gas was drawn, the esophageal pressure recorded, and the difference between end-expiratory lung volume and equilibrium volume measured by exposing the ETT and the negative pressure chamber to atmospheric pressure and permitting passive lung emptying. This exhaled volume was termed EELV, neglecting any small volume of residual gas at equilibrium. Between applications of constant pressure, tidal ventilation was provided (positive pressure, V T 9 mL/kg, PEEP 10 cmH2O). After completion of the randomized series of positive (or negative) pressure distensions, the alternative mode (i.e., positive vs. negative) was utilized; then the sequence of P L exposures was again randomized.
To ensure a consistent lung volume history, an identical recruitment maneuver (negative pressure, −20, then −10, and then −5 cmH2O, each for 10 s) was applied before each sustained positive pressure or negative pressure distension.
In vivo rabbit, cyclic inflation (surfactant-depleted)
Ten New Zealand white rabbits (2.5–3.0 kg; Charles River; QC, Canada) were anesthetized, instrumented, and surfactant depleted as above. Each animal was randomized to receive either positive or negative ventilation and then received injurious ventilation (12 mL/kg, FiO2 1.0, end-expiratory pressure (EEP) of 5 cmH2O) for 30 min [6]. This (arbitrary) ventilation time period does not produce significant lung injury. EEPs of 4, 6, 8, and 10 cmH2O were then established and maintained for 20 min each. After 20 min P L, PaO2, and EELV were recorded [6]. The EELV was measured by occluding the endotracheal tube at end-expiration, discontinuing ventilation, releasing the occlusion, and measuring the volume of exhaled gas with a spirometer (Bear Neonatal Volume Monitor NVM1: Bear Medical Systems, Riverside, CA) until flow ceased.
Statistical analysis
Data are expressed as mean ± standard deviation, and analysis used analysis of variance (ANOVA), with post hoc Student–Neuman Keuls testing. Statistical significance was inferred where P < 0.05, and SigmaStat™ (Version 3.1; Systat Software Inc., San Jose, CA, USA) was used for calculations.
Results
Pressure–time profile, isolated mouse lungs (ex vivo)
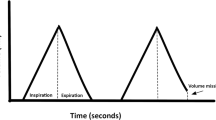
Sample inflation pressure profiles were obtained with positive and negative tidal ventilation demonstrating matching of the pressure–time profiles in positive versus negative pressure ventilation (Fig. 1a); the two profiles were superimposable (Fig. 1b). Isolated mouse lungs randomly allocated to dynamic ventilation with matched (see above) positive or negative pressure ventilation (peak pressures 7.5, 10, or 15 cmH2O) yielded identical tidal volumes (Fig. 1c).
Tidal ventilation in ex vivo mouse lungs: cyclic ventilation using reciprocal and identical pressure–time profiles with positive and negative pressure inflation yields similar volume–time profiles (a) that can be superimposed (b). Cyclic ventilation using positive versus negative inflation pressures of 5, 7.5, and 10 cmH2O resulted in identical mean tidal volumes at each level of inflation pressure (c)
Tidal ventilation, intact mouse (in vivo)
Tidal ventilation achieved with pressure ventilation at peak inflation pressures of 7.5, 10, and 15 cmH2O resulted in identical tidal volumes whether driven by positive or negative pressure ventilation (Fig. 2).
Tidal ventilation of in vivo mouse lungs: cyclic ventilation using reciprocal and identical pressure–time profiles with positive and negative pressure inflation yields similar mean tidal volumes with peak inspiratory inflation pressures of 5, 7.5, and 10 cmH2O. End-expiratory pressures of 3 cmH2O were used in all cases. EEP end-expiratory pressure
Sustained inflation, surfactant-depleted rabbit (in vivo)
This preparation allowed serial measurement of EELV and arterial blood gases in vivo. At applied distending pressures of 5, 10, 15, or 20 cmH2O, the relationship between EELV and arterial oxygenation was similar whether inflation was achieved with positive versus negative pressure inflation (Fig. 3).
Cyclic inflation, surfactant-depleted rabbit (in vivo)
Surfactant-depleted rabbits were used to compare positive versus negative pressure square-wave ventilation in a repeat of previous in vivo studies, but with better matched pressure–time profiles, and the results compared with historical negative ventilation data [6]. Well-matched positive and negative ventilation yielded similar values for PaO2 at all levels of EELV. However, the historical data [6] with less well matched negative ventilation yielded higher values for PaO2 (P < 0.03, ANOVA on ranks) in the mid-range—but not at extremes—of EELV (Fig. 4).
Tidal ventilation of in vivo surfactant-depleted rabbit lungs: PaO2 was similar at various levels of EELV following comparable (square-wave) positive versus negative pressure ventilation. In contrast, comparison with historical negative pressure data where the pressure–time profile was non-square-wave (and non-identical), demonstrated higher PaO2 at mid-range levels of EELV (*P < 0.03, ANOVA)
Discussion
These data demonstrate that ventilation with positive and negative pressure results in similar dynamic and static lung volumes. The findings have been corroborated using ex vivo as well as in vivo normal and surfactant-depleted lungs. Where positive and negative pressure ventilation was used to achieve similar in vivo lung volumes, the resultant oxygenation was similar.
Previous reports of positive versus negative pressure ventilation
Some of the current findings stand in contrast to previous reports where negative pressure was associated with less lung injury, improved oxygenation, and preferential recruitment (i.e., more reversal of atelectasis, less expansion of dead space) [6, 13], but are consistent with studies of in vivo lung injury that reported no difference in net impact of the two modes of ventilation [14]. Some features of study design may explain differences between the earlier [6] and the current data. For example, in the initial report [6], matching the pressure–time waveform in the positive and the negative pressure experiments was attempted but was limited by equipment performance. However, in the current report, the dynamic ex vivo and murine—very small animal—in vivo (Figs. 1c and 4, respectively) experiments utilized apparatus with the ability to match identically pressure–time profiles for positive and negative pressure. With positive pressure ventilation, the pressure–time profile reflects a balance between the pattern of inspiratory fresh gas flow entering the circuit (and airways) and the total volume of the apparatus plus the airways. In contrast, with negative pressure ventilation, considerable flows would be required to rapidly evacuate the volume of the chamber (especially in the initial inspiration) to match the flows used with the positive pressure apparatus. In the current experiments, high-performance negative pressure devices allowed superimposable matching of pressure–time profiles (Figs. 1, 2). Thus the dynamic studies were performed in apparatus with excellent dynamic response characteristics, and therefore a lack of difference in tidal volumes across a series of positive versus negative pressure experiments (where successful matching of the pressure–time profile was accomplished) supports the idea that there is a comparable impact of positive versus negative pressure ventilation.
While matching the pressure–time profile for positive and negative pressure can be challenging for dynamic studies, it is far less so for static conditions. In the previous studies the pressure–time profiles were matched (positive versus negative) under static conditions, yet the oxygenation [6] and CT recruitment characteristics [13] were superior in the setting of negative pressure ventilation, suggesting that additional influences must have affected lung inflation. In this context, the lung volume history may well be important. In the previous studies—where negative pressure was associated with improved oxygenation—the lung volume history may have differed between the positive and negative pressure strategies. In the current series, all preparations were exposed to identical lung volume history before exposure to static positive or negative pressure distension, and this resulted in identical oxygenation profiles (Fig. 3).
Approaches to negative pressure ventilation
Negative pressure ventilation can be applied to the chest wall, the chest and abdominal wall together, or selectively to the abdomen. Application to the chest is usually employed for long-term (e.g., domiciliary) ventilation, and several devices (e.g., cuirass) have been reported [9]. Use of negative pressure ventilation directed to the entire body (except the head and neck) originally involved the iron lung for supportive mechanical ventilation. Cyclic [9] and continuous [15] negative pressure ventilation can be effective for maintaining or supporting ventilation, although usually not in the presence of significant lung injury.
Understanding the anatomical area over which negative pressure is applied may be important. Indeed, negative pressure ventilation has been applied with the aim of selectively recruiting basal atelectasis in a porcine model [16] and in patients with ARDS [10]. Their approach was to apply negative pressure selectively to the anterior abdominal wall, as an adjunct to ongoing (conventional) positive pressure ventilation and while this likely resulted in reduced abdominal pressure, it did not effectively transfer distending pressure into atelectatic lung. It is possible that this approach was ineffective because of the selective application to the upper anterior abdominal wall as opposed to the whole abdomen.
Mechanical ventilation also has important hemodynamic effects. Negative pressure applied only to the chest increases venous return and augments ventricular filling [17], which in the absence of impaired ventricular function, increases cardiac output; positive pressure ventilation has the opposite effect on venous return, and can reduce cardiac output despite reducing ventricular afterload. Applying negative pressure simultaneously to the chest and the abdomen results in minimal net effect on venous return, as was demonstrated by thermodilution measurements in the in vivo rabbit in a model identical to that used in the current study [6].
Theoretical comparison: positive versus negative pressure
While the ‘net’ effects of negative and positive pressure are equivalent in terms of transpulmonary pressure generated, there are theoretical reasons for some differences in impact. Within homogeneous lung, the distributions of alveolar pressures are reasonably uniform and thus local stresses and volumes would be determined by transpulmonary pressure at end-exhalation (Fig. 5a) and at end-inspiration. Tidal gas exchange (in moles of gas) would be increased by gas compression during positive pressure but decreased by rarefaction during negative pressure ventilation, resulting in up to 4% greater tidal gas exchange during the former for a given geometrical volume change.
Schematic of positive versus negative pressure ventilation under quasistatic conditions: initial conditions at end-exhalation (a); positive pressure ventilation with 40 cmH2O of airway pressure applied (b); and negative pressure ventilation with −40 cmH2O pressure applied to the body surface (c). For full description see Appendix C (supplementary material)
However, within heterogeneous lung, some lung units may communicate with the central airways, whereas other lung units could be non-communicating (e.g., airway occlusion; Fig. 5b, c). In such a circumstance, positive pressure inflation (Fig. 5b) would be predicted to raise pleural pressure via inflation of communicating alveoli, with the resulting pleural pressure change exerting a compressive effect on the occluded lung unit and reducing its volume. Indeed recent studies of alveolar micromechanics support this concept where fluid filling causes alveoli to shrink and the adjacent (air-filled) alveoli to expand [18]. However, negative pressure inflation (Fig. 5c) would result in rarefaction—and not compression—of the gas in the occluded lung unit. We have made quantitative estimates of the impact of gas compression/rarefaction based on theoretical assumptions and have quantified a small effect (<3%) of negative versus positive pressure ventilation on geometric volume of the non-communicating lung units (see Appendix B (supplementary material)). In the non-communicating lung regions, such a geometrical volume difference has no influence on the tidal exchange of gas or overall gas exchange, although it may contribute to differences in local strains in generating stress. Finally, neither positive nor negative pressure will impact on a non-inflatable atelectatic lung unit.
In summary, there is a theoretical small decrease in volume of an obstructed lung unit during positive pressure inflation (Fig. 5b), in contrast to a small increase in volume in an obstructed lung unit during negative pressure inflation (Fig. 5c). See Appendix B (supplementary material) for quantitative estimates.
The critical variable governing these interactions is pleural pressure, which is a function of the relative lung and chest wall compliance. As such, the theoretical differences between negative and positive pressure ventilation are quantitatively small even within heterogeneous, injured lung. However, the degree to which even small regional differences in alveolar pressure could contribute to high local shear forces on the lung is unclear, particularly because in lung injury (e.g., ARDS) the effects are heterogeneous and the geometry is complex.
Limitations
The steady-state measurements employed do not reflect physiologic conditions and were not models of established lung injury. In addition, lung injury in humans, which develops over days, is associated with more complex inhomogeneity than is illustrated here. While the theoretical model addresses parallel heterogeneity it does not address serial heterogeneity, such as the impact of extrathoracic airways. Finally, the current study does not take into account how spontaneous ventilation efforts (that can develop considerable negative pleural pressure) could affect both positive and negative pressure ventilation.
Conclusions
In ex vivo and in vivo experimental lung models, dynamic and static inflation were used to compare positive versus negative pressure ventilation. Where negative pressure was globally applied, i.e., to the whole lung (ex vivo) (or complete chest and abdomen; in vivo) and where both the lung volume history and the pressure–time profiles were matched, we report no differences in the effects of positive versus negative pressure ventilation on lung volumes or oxygenation. We present a theoretical argument applicable to heterogeneous and partially obstructed lungs that could account for very small differences, where ventilation with positive pressure might produce slightly smaller (ca. 3–4%) changes in geometrical volume than with negative pressure. On the basis of the current data, we believe that a simple comparison of clinical outcomes with positive versus negative pressure ventilation is not warranted.
References
Borelli M, Benini A, Denkewitz T, Acciaro C, Foti G, Pesenti A (1998) Effects of continuous negative extrathoracic pressure versus positive end-expiratory pressure in acute lung injury patients. Crit Care Med 26:1025–1031
Lockhat D, Langleben D, Zidulka A (1992) Hemodynamic differences between continual positive and two types of negative pressure ventilation. Am Rev Respir Dis 146:677–680
Shekerdemian LS, Schulze-Neick I, Redington AN, Bush A, Penny DJ (2000) Negative pressure ventilation as haemodynamic rescue following surgery for congenital heart disease. Intensive Care Med 26:93–96
Easa D, Mundie TG, Finn KC, Hashiro G, Balaraman V (1994) Continuous negative extrathoracic pressure versus positive end-expiratory pressure in piglets after saline lung lavage. Pediatr Pulmonol 17:161–168
Corrado A, Ginanni R, Villella G, Gorini M, Augustynen A, Tozzi D, Peris A, Grifoni S, Messori A, Nozzoli C, Berni G (2004) Iron lung versus conventional mechanical ventilation in acute exacerbation of COPD. Eur Respir J 23:419–424
Grasso F, Engelberts D, Helm E, Frndova H, Jarvis S, Talakoub O, McKerlie C, Babyn P, Post M, Kavanagh BP (2008) Negative-pressure ventilation: better oxygenation and less lung injury. Am J Respir Crit Care Med 177:412–418
Green M, Mead J (1974) Time dependence of flow-volume curves. J Appl Physiol 37:793–797
Corrado A, Gorini M, Villella G, De Paola E (1996) Negative pressure ventilation in the treatment of acute respiratory failure: an old noninvasive technique reconsidered. Eur Respir J 9:1531–1544
Jackson M, Kinnear W, King M, Hockley S, Shneerson J (1993) The effects of five years of nocturnal cuirass-assisted ventilation in chest wall disease. Eur Respir J 6:630–635
Valenza F, Bottino N, Canavesi K, Lissoni A, Alongi S, Losappio S, Carlesso E, Gattinoni L (2003) Intra-abdominal pressure may be decreased non-invasively by continuous negative extra-abdominal pressure (NEXAP). Intensive Care Med 29:2063–2067
Jaecklin T, Engelberts D, Otulakowski G, O’Brodovich H, Post M, Kavanagh BP (2011) Lung-derived soluble mediators are pathogenic in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 300:L648–L658
Murphy DB, Cregg N, Tremblay L, Engelberts D, Laffey JG, Slutsky AS, Romaschin A, Kavanagh BP (2000) Adverse ventilatory strategy causes pulmonary-to-systemic translocation of endotoxin. Am J Respir Crit Care Med 162:27–33
Helm E, Talakoub O, Grasso F, Engelberts D, Alirezaie J, Kavanagh BP, Babyn P (2009) Use of dynamic CT in acute respiratory distress syndrome (ARDS) with comparison of positive and negative pressure ventilation. Eur Radiol 19:50–57
Dreyfuss D, Saumon G (1993) Role of tidal volume, FRC, and end-inspiratory volume in the development of pulmonary edema following mechanical ventilation. Am Rev Respir Dis 148:1194–1203
Morris AH, Elliott CG (1985) Adult respiratory distress syndrome: successful support with continuous negative extrathoracic pressure. Crit Care Med 13:989–990
Valenza F, Irace M, Guglielmi M, Gatti S, Bottino N, Tedesco C, Maffioletti M, Maccagni P, Fossali T, Aletti G, Gattinoni L (2005) Effects of continuous negative extra-abdominal pressure on cardiorespiratory function during abdominal hypertension: an experimental study. Intensive Care Med 31:105–111
Skaburskis M, Helal R, Zidulka A (1987) Hemodynamic effects of external continuous negative pressure ventilation compared with those of continuous positive pressure ventilation in dogs with acute lung injury. Am Rev Respir Dis 136:886–891
Perlman CE, Lederer DJ, Bhattacharya J (2011) Micromechanics of alveolar edema. Am J Respir Cell Mol Biol 44:34–39
Lai-Fook SJ, Hyatt RE, Rodarte JR (1978) Effect of parenchymal shear modulus and lung volume on bronchial pressure-diameter behavior. J Appl Physiol 44:859–868
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Engelberts, D., Malhotra, A., Butler, J.P. et al. Relative effects of negative versus positive pressure ventilation depend on applied conditions. Intensive Care Med 38, 879–885 (2012). https://doi.org/10.1007/s00134-012-2512-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2512-5