Abstract
Purpose
Near-infrared spectroscopy has been used as a noninvasive monitoring tool for tissue oxygen saturation (StO2) in acutely ill patients. This study aimed to investigate whether local vasoconstriction induced by body surface cooling significantly influences thenar StO2 as measured by InSpectra model 650.
Methods
Eight healthy individuals (age 26 ± 6 years) participated in the study. Using a cooling blanket, we aimed to cool the entire body surface to induce vasoconstriction in the skin without any changes in central temperature. Thenar StO2 was noninvasively measured during a 3-min vascular occlusion test using InSpectra model 650 with a 15-mm probe. Measurements were analyzed for resting StO2 values, rate of StO2 desaturation (RdecStO2, %/min), and rate of StO2 recovery (RincStO2, %/s) before, during, and after skin cooling. Measurements also included heart rate (HR), mean arterial pressure (MAP), cardiac output (CO), stroke volume (SV), capillary refill time (CRT), forearm-to-fingertip skin-temperature gradient (Tskin-diff), perfusion index (PI), and tissue hemoglobin index (THI).
Results
In all subjects MAP, CO, SV, and core temperature did not change during the procedure. Skin cooling resulted in a significant decrease in StO2 from 82% (80–87) to 72% (70–77) (P < 0.05) and in RincStO2 from 3.0%/s (2.8–3.3) to 1.7%/s (1.1–2.0) (P < 0.05). Similar changes in CRT, Tskin-diff, and PI were also observed: from 2.5 s (2.0–3.0) to 8.5 s (7.2–11.0) (P < 0.05), from 1.0°C (−1.6–1.8) to 3.1°C (1.8–4.3) (P < 0.05), and from 10.0% (9.1–11.7) to 2.5% (2.0–3.8), respectively. The THI values did not change significantly.
Conclusion
Peripheral vasoconstriction due to body surface cooling could significantly influence noninvasive measurements of thenar StO2 using InSpectra model 650 with 15-mm probe spacing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Near-infrared spectroscopy (NIRS) is a noninvasive technique that allows the determination of tissue oxygenation based on spectrophotometric quantitation of oxy- and deoxyhemoglobin levels within a tissue. Since its advent as a noninvasive monitoring tool for peripheral tissue oxygenation, a relationship between the potential influences of skin circulation on tissue oxygen saturation (StO2) signals has been debated. Numerous studies have investigated different NIRS devices in various tissues and under various experimental conditions [1–4]. These studies indicate that the StO2 signal is widely influenced by the volume of the vascular bed in the catchment area of the probe. Current clinical NIRS studies, particularly those performed in an intensive care setting seem to neglect this relationship when monitoring peripheral tissue oxygenation. One of the reasons may be the lack of studies that address the influence of thenar skin circulation on StO2 signal as measured with the most current commercial device available (InSpectra model 650).
We recently reported in an observational study that abnormalities in skin perfusion contribute significantly to the StO2-derived signal measured with an InSpectra model 650 probe on the thenar, and this correlation was independent of disease condition or systemic hemodynamics [5]. The question that remains is how important is the potential contribution of low skin blood flow, specifically of the thenar eminence, on StO2? To answer this question, we performed StO2-derived tissue oxygenation measurements during local vasoconstriction induced by extremity cooling. We hypothesize that the decrease in skin blood flow resulting from peripheral vasoconstriction during body surface cooling significantly influences the noninvasive measurement of thenar StO2 using an InSpectra model 650 with 15-mm probe spacing.
Materials and methods
Study population
The study was conducted at a university-affiliated teaching hospital. We recruited healthy volunteers with no history of receiving any vasoactive medication. The volunteers were instructed to avoid caffeine-containing drinks for 24 h before the experiments. The local medical ethics committee approved this study protocol.
Measurements
StO2-derived tissue oxygenation
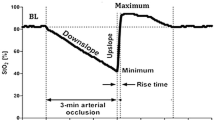
StO2-derived tissue oxygenation was continuously monitored using an InSpectra tissue spectrometer model 650 with a 15-mm probe over the thenar eminence. A vascular occlusion test (VOT) was performed by arrest of forearm blood flow using a conventional sphygmomanometer pneumatic cuff. The cuff was placed around the upper arm and was inflated to a pressure approximately 30 mmHg greater than patient systolic pressure for 3 min. On the completion of the ischemic period, the occluding cuff was rapidly deflated to 0 mmHg. VOT-derived StO2 parameters were divided into three components: resting StO2 values, rate of StO2 desaturation (RdecStO2, expressed as %/min), and rate of StO2 recovery (RincStO2, expressed as %/s).
Peripheral perfusion
Peripheral perfusion was evaluated using conventional physical examination with capillary refill time (CRT), forearm-to-fingertip skin-temperature gradient (Tskin-diff), perfusion index (PI), and tissue hemoglobin index (THI). CRT was measured by applying firm pressure to the distal phalanx of the index finger for 15 s. A chronometer recorded the time for the return of the normal color. The Tskin-diff was obtained with two skin probes (Hewlett Packard 21078A) attached to the index finger and on the radial side of the forearm, midway between the elbow and the wrist. Tskin-diff can better reflect changes in cutaneous blood flow than skin temperature itself. When being evaluated under constant environmental conditions, Tskin-diff increases during vasoconstriction, and a threshold of 4°C has been shown to reflect vasoconstriction in critically ill patients [6]. The PI provides a noninvasive method for evaluating perfusion and has been shown to reflect changes in peripheral perfusion [7]. In this study, the PI value was obtained using Masimo pulse oximetry, which displays a range from 0.02% (very weak pulse strength) to 20% (very strong pulse strength). The THI was derived from a second-derivative attenuation spectrum and is part of the StO2 algorithm of the NIRS monitor.
Global hemodynamic parameters
Global hemodynamic parameters included heart rate (HR), stroke volume (SV), cardiac output (CO), and mean arterial pressure (MAP). Global parameters were recorded using thoracic bioimpedance, as measured by a noninvasive cardiac output monitor (NICOM; Cheetah Medical Inc., Wilmington, DE, USA). The NICOM system and technology have been described elsewhere [8]. In summary, connecting the NICOM to the subject requires four double electrode stickers placed on the thorax, according to the manufacturer’s instructions. Data are automatically recorded using a computer data logger on a minute-by-minute basis.
Cooling techniques and monitoring
Body surface cooling was achieved using circulating cold water blankets (Thermowrap, Or-Akiva Ind. Park, Israel). A cooling pump device (CSZ Blanketrol III, model 233, Cincinnati SubZero, Inc.) was connected to the blankets to pump cold water. The water temperature was set to the desired temperature. The blanket garment was attached directly to the patient’s body using medically approved adhesive.
Protocol
Individuals were positioned in supine position dressed with the cooling blankets on a comfortable bed. The blanket suit covered the entire body with the exception of the head, instrumented forearm, hands, and feet. The cooling pump device permitted control of blanket water temperature by changing the temperature of water perfusing the suit. The suit was then perfused with 32°C water. Electronic measurements were obtained continuously and the values are reported as averaged data for each interval. The time points included the following: baseline measurements prior to the cooling process (T 0), after 30 min of peripheral cooling (T 1), and after 30 min of the suspension of cooling and initiation of the rewarming process (T 2). For this protocol, peripheral cooling was designed mainly to chill the skin over the entire body to induce only skin vasoconstriction without any changes in central temperature. Therefore, core temperature was measured each 5 min with an infrared tympanic thermometer (First Temp Genius model 3000A). Ambient temperature was constant during all experiments (T = 22°C). Skin vasoconstriction was defined as a minimal 50% decrease either in the Tskin-diff temperature or the PI signal.
Statistical analysis
The results are presented as the median (25th–75th), unless otherwise specified. A one-way repeated-measures ANOVA was conducted to compare NIRS-derived and peripheral perfusion parameters prior, during, and after rewarming. The Bonferroni post hoc test was performed if a significant main effect was observed. SPSS (version 15.0, SPSS, Chicago, IL) was used for statistical analysis. A P value less than 0.05 was considered statistically significant.
Results
Eight healthy individuals (4 male, 4 female) participated in the study. The mean age, height, and weight were 26 ± 6 years, 172 ± 5 cm, and 74.1 ± 6.2 kg, respectively. Table 1 lists the global hemodynamic variables stratified by the time points of the study. All subjects tolerated the cooling process well and did not develop shivering during the experiment. We found a nonsignificant tendency towards an increase in SV and CO during peripheral cooling at T 1 (Table 1). Core temperature and heart rate did not change significantly during the experiment.
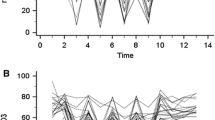
Figure 1 presents the time course of the NIRS dynamic variables and peripheral perfusion parameters before, during, and after the skin cooling process. Table 2 lists the absolute values of all peripheral parameters as stratified by time points. The peripheral cooling resulted in skin vasoconstriction in all volunteers with a significant decrease in the Tskin-diff temperature and in the PI signal: 55% (40–175) and 77% (62–83) compared with baseline, respectively. Concomitantly, we observed a significant decrease in StO2 and RincStO2 values but not in RdecStO2. The rewarming process increased StO2 and RincStO2 values towards baseline levels. Similar changes in CRT, Tskin-diff, and PI were also observed. The THI values did not change significantly during the entire experiment.
Time course of NIRS-derived variables and peripheral perfusion parameters. T 0, prior to the cooling process; T 1, after 30 min of peripheral cooling; T 2, after 30 min of the suspension of cooling and initiation of the rewarming process. a StO2, peripheral tissue oxygenation (%); b RincStO2, rate of StO2 recovery after arterial occlusion (%/s); c RdecStO2, rate of StO2 desaturation during arterial occlusion (%/min); d Tskin-diff, forearm-to-fingertip skin-temperature gradient (°C); e PI, perfusion index (%); f CRT, capillary refill time (s). Lines represent individual values for each healthy volunteer. Bars are mean ± 95% CI
We were also interested in investigating which of the NIRS variables was most affected by changes in skin circulation. Our results indicated that the magnitude of changes seem to be more prominent in RincStO2 than StO2 and that RincStO2 is more sensitive to changes in peripheral perfusion than StO2 itself. When compared with baseline values, the magnitude of the RincStO2 decreases was larger than that observed for StO2 [47% (30–62%) vs. 11% (9.2–13.1), P = 0.001].
Discussion
The key finding from this study is that changes in vasomotor tone in the skin of the thenar eminence contributed significantly to the StO2-derived parameters as measured with a NIRS InSpectra device. The main mechanistic theory of our study is that peripheral vasoconstriction due to surface cooling results in decreased perfusion of the skin and, therefore, in parallel, changes in the StO2 resting values and in the StO2 recovery rate. Under resting conditions, the impact of peripheral perfusion alterations on NIRS-derived measurements can be expected to be magnified as the skin temperature decreases.
This finding was not totally unexpected because light from the NIRS system must pass through the skin and some absorbance in the resistance vessels that supply subepidermal capillaries would be anticipated. The 15-mm NIRS probe mainly covers approximately an 8-mm depth of tissue and focusing on the muscle. Skin and subcutaneous layers above the muscle definitely contribute to the overall StO2 measured. It is likely that the decreasing StO2 effect after skin cooling is mainly due to the upper layers’ compromised perfusion because of cutaneous vasoconstriction. One may argue that it is possible that the cooling device induced changes beyond that of skin circulation and that flow in skeletal muscle was also altered. Our study does not allow us to conclude which of the two components (skin or muscle) is the major contributor to the changes in StO2-derived variables in our model. Nevertheless, we speculate that participation of muscle blood flow was not predominant because THI readings in our volunteers presented small changes during the cooling period. On the other hand, participation of skin blood flow was significant, as reflected by changes in skin temperature, PI, and CRT. The THI represents the total tissue concentration of hemoglobin in both extravascular and vascular tissue, and its physiological significance and clinical utility are still under investigation. Our model induced changes mainly on the arterial side of microcirculation and may explain why THI was not affected by the peripheral cooling device, as the sensitivity of THI is greater for vessels with high capacitance, such as post capillaries and venous compartments. This phenomenon may explain why other NIRS researchers have reported low THI values and normal StO2 in patients with sepsis, as this is a condition related to vascular leak and low vascular density due to microcirculatory derangements [9, 10]. Therefore, the decreased NIRS signal from oxygenated hemoglobin is likely the result of a decrease in arterial blood volume within the peripheral vasculature as a consequence of vasoconstriction.
We chose this model to test our hypotheses because previous studies, including one by our own group, have shown that peripheral vasoconstriction is a frequent abnormality in critically ill patients [11, 12]. For instance, studies that employed NIRS as a peripheral tissue oxygenation monitoring device have shown that the fall in StO2 in peripheral tissues correlates well with the degree of hypotension in trauma and hemorrhagic shock [13–16]. However, these findings were always related to acute shock states and the disturbance of the systemic circulation, which indicates that the pathological link between hypotension and the fall in StO2 may be explained by increased peripheral vasoconstriction as a result of the adrenergic response that follows the neurohumoral compensatory mechanisms induced by the low-flow systemic shock state. Peripheral vasoconstriction may very well explain the fall in StO2 levels in acute situations, such as in trauma and cardiogenic or hemorrhagic shock. In critically ill patients after resuscitation of the systemic circulation, during the stability phase, peripheral vascular tone may no longer reflect the acute compensatory mechanisms because others factors overcome this physiologic response; such factors include mechanical ventilation, vasopressors, vasodilators, sedatives, and opiate use. However, abnormalities in peripheral perfusion may persist despite patient systemic hemodynamic stability [11, 12, 17]. The noticeable decreases in StO2 and StO2 recovery rate in our model provide evidence that peripheral vasoconstriction markedly influences the NIRS measurements of thenar tissue oxygenation and may confound interpretation of StO2-derived parameters in critically ill patients, in whom peripheral perfusion constantly changes over time.
Another interesting finding was that we could induce significant changes in StO2-derived tissue oxygenation values by maintaining unchanged systemic hemodynamics. We found a nonsignificant tendency towards an increase in SV and CO during peripheral cooling. This finding may be explained by the shift of blood volume from the vasoconstricted peripheral circulation to the central circulation with a subsequent increase in the cardiac preload, justifying the augmentation of cardiac output as a result of an increase in stroke volume. In one previous study conducted by our group, we found that changes in StO2-derived parameters were correlated with parameters of peripheral perfusion in critically ill patients but were independent of the hemodynamic status of the patient [5]. In another recent study in a model of controlled central hypovolemia, a decreased venous return with a concomitant decrease in stroke volume did not lead to clinically significant changes in StO2 as measured on the thenar [18]. These findings strongly suggest that StO2-derived parameters are more affected by changes in peripheral vasomotor tone than by systemic hemodynamic conditions. Perhaps even more interesting to the clinician who applies NIRS for peripheral perfusion monitoring is the knowledge that abnormal StO2-derived parameters may reflect a condition of peripheral vasoconstriction independent of systemic hemodynamics.
In conclusion, the presence of peripheral vasoconstriction due to body surface cooling could significantly influence the noninvasive measurement of thenar StO2 using an InSpectra model 650 with 15-mm probe spacing. Depending on the condition of peripheral circulation, significant decreases in peripheral blood flow can affect StO2-derived measurements, particularly StO2 and StO2 recovery rate, which are exclusively dependent on local vasodilation capacity. Therefore, careful consideration must be given when using NIRS to measure tissue oxygenation in critically ill patients, and consideration should be given to the peripheral circulation when interpreting peripheral tissue oxygenation.
References
Buono MJ, Miller PW, Hom C, Pozos RS, Kolkhorst FW (2005) Skin blood flow affects in vivo near-infrared spectroscopy measurements in human skeletal muscle. Jpn J Physiol 55:241–244
Takahashi T, Takikawa Y, Kawagoe R, Shibuya S, Iwano T, Kitazawa S (2011) Influence of skin blood flow on near-infrared spectroscopy signals measured on the forehead during a verbal fluency task. Neuroimage 57:991–1002
De Blasi RA (2008) Is muscle StO2 an appropriate variable for investigating early compensatory tissue mechanisms under physiological and pathological conditions? Intensive Care Med 34:1557–1559
Yanagisawa O, Homma T, Okuwaki T, Shimao D, Takahashi H (2007) Effects of cooling on human skin and skeletal muscle. Eur J Appl Physiol 100:737–745
Lima A, van Bommel J, Sikorska K, van Genderen M, Klijn E, Lesaffre E, Ince C, Bakker J (2011) The relation of near-infrared spectroscopy with changes in peripheral circulation in critically ill patients. Crit Care Med 39:1649–1654
Sessler DI (2003) Skin-temperature gradients are a validated measure of fingertip perfusion. Eur J Appl Physiol 89:401–402
Lima AP, Beelen P, Bakker J (2002) Use of a peripheral perfusion index derived from the pulse oximetry signal as a noninvasive indicator of perfusion. Crit Care Med 30:1210–1213
Squara P, Denjean D, Estagnasie P, Brusset A, Dib JC, Dubois C (2007) Noninvasive cardiac output monitoring (NICOM): a clinical validation. Intensive Care Med 33:1191–1194
Skarda DE, Mulier KE, Myers DE, Taylor JH, Beilman GJ (2007) Dynamic near-infrared spectroscopy measurements in patients with severe sepsis. Shock 27:348–353
Mulier KE, Skarda DE, Taylor JH, Myers DE, McGraw MK, Gallea BL, Beilman GJ (2008) Near-infrared spectroscopy in patients with severe sepsis: correlation with invasive hemodynamic measurements. Surg Infect (Larchmt) 9:515–519
Lima A, Jansen TC, van Bommel J, Ince C, Bakker J (2009) The prognostic value of the subjective assessment of peripheral perfusion in critically ill patients. Crit Care Med 37:934–938
Kaplan LJ, McPartland K, Santora TA, Trooskin SZ (2001) Start with a subjective assessment of skin temperature to identify hypoperfusion in intensive care unit patients. J Trauma 50:620–627
McKinley BA, Marvin RG, Cocanour CS, Moore FA (2000) Tissue hemoglobin O2 saturation during resuscitation of traumatic shock monitored using near infrared spectrometry. J Trauma 48:637–642
Crookes BA, Cohn SM, Bloch S, Amortegui J, Manning R, Li P, Proctor MS, Hallal A, Blackbourne LH, Benjamin R, Soffer D, Habib F, Schulman CI, Duncan R, Proctor KG (2005) Can near-infrared spectroscopy identify the severity of shock in trauma patients? J Trauma 58:806–813
Ikossi DG, Knudson MM, Morabito DJ, Cohen MJ, Wan JJ, Khaw L, Stewart CJ, Hemphill C, Manley GT (2006) Continuous muscle tissue oxygenation in critically injured patients: a prospective observational study. J Trauma 61:780–788
Cohn SM, Nathens AB, Moore FA, Rhee P, Puyana JC, Moore EE, Beilman GJ (2007) Tissue oxygen saturation predicts the development of organ dysfunction during traumatic shock resuscitation. J Trauma 62:44–54
Thompson MJ, Ninis N, Perera R, Mayon-White R, Phillips C, Bailey L, Harnden A, Mant D, Levin M (2006) Clinical recognition of meningococcal disease in children and adolescents. Lancet 367:397–403
Bartels SA, Bezemer R, de Vries FJ, Milstein DM, Lima A, Cherpanath TG, van den Meiracker AH, van Bommel J, Heger M, Karemaker JM, Ince C (2011) Multi-site and multi-depth near-infrared spectroscopy in a model of simulated (central) hypovolemia: lower body negative pressure. Intensive Care Med 37:671–677
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Lima, A., van Genderen, M.E., Klijn, E. et al. Peripheral vasoconstriction influences thenar oxygen saturation as measured by near-infrared spectroscopy. Intensive Care Med 38, 606–611 (2012). https://doi.org/10.1007/s00134-012-2486-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2486-3