Abstract
Purpose
The aim of this study is to examine long-term pulmonary function and quality of life in survivors of acute respiratory distress syndrome (ARDS) previously enrolled in a randomized multicenter trial testing prone compared with supine positioning (PSII study) at five Italian centers.
Design
Observational prospective study.
Subjects and measurements
Pulmonary function [spirometric test, gas exchange, carbon monoxide diffusion capacity (DLCO)], high-resolution computed tomography (CT) scan, and health-related quality of life [Short Form-36 (SF-36) and St. George’s Respiratory Questionnaire] were evaluated at 12 months.
Results
Twenty-six patients (13 in each group, mean age 54.1 ± 2.8 years, body mass index 24.5 ± 1.4 kg/m2, PaO2/FiO2 117 ± 49 mmHg) were evaluated. There were no significant differences in demographic data, illness severity, or outcome between the prone and supine groups. The overall survival rate was 40%. Pulmonary function was in the normal range without any differences between the two groups. Quantitative lung CT scan analysis showed similar amounts for not aerated (8.1 ± 3.2% versus 7.3 ± 3.4%), poorly aerated (15.3 ± 3.6% versus 17.1 ± 4.9%), and well-aerated (64.0% ± 8.4 versus 70.2 ± 8.4%) lung regions, while overaerated lung region was slightly higher in the prone compared with the supine group (12.5 ± 6.5% versus 5.3 ± 5.5%). Health-related quality of life was similar to in healthy population. However, these patients showed reduction in daily activity specifically due to pulmonary disease as measured by the St. George’s Respiratory Questionnaire.
Conclusions
No differences in pulmonary function or quality of life were observed in this small group of ARDS survivor patients treated in prone versus supine position.
Similar content being viewed by others
Introduction
Acute respiratory failure (ARF), caused by acute respiratory distress syndrome (ARDS) and acute lung injury (ALI), is a rapidly progressive clinical condition characterized by impairment of gas exchange which requires mechanical ventilation [1]. Mechanical ventilation may also cause several types of lung damage called ventilator-induced lung injury [2]. Despite a trend toward better outcome over the years, the mortality rate in ARDS remains above 40% [3], with elevated morbidity [4, 5] and social costs [6]. Moreover, it was previously found that surviving ARDS patients may present persistent functional limitation [4, 7–10] and reduction in health-related quality of life (HRQL) [5, 10–13].
Prone position has been suggested to improve gas exchange and reduce ventilator-induced lung injury [14] by inducing a more homogeneous distribution of lung stress and strain [15–18]. Although these studies showed that, in the whole population of ALI/ARDS patients, prone position did not show any benefit in outcome [19–22], in the more hypoxemic patients a significant reduction of mortality of about 10% compared with supine position has been noted [19, 21–23].
In conjunction with a randomized multicenter trial that evaluated long-term use of prone compared with supine position in a population of ARDS patients [22], we aimed to investigate in surviving patients whether prone positioning presents any advantage in terms of pulmonary function and quality of life compared with supine positioning.
Subjects and methods
This report details long-term outcomes of ARDS patients enrolled in a randomized multicenter trial (PSII study) [22] investigating possible outcome benefits of prone positioning. There were no significant differences in mortality rate between prone versus supine patients at 28 days, at intensive care discharge, or at 6 months [22]. Patients enrolled in the PSII study fulfilled the diagnostic criteria of ARDS, i.e., PaO2/FiO2 ratio ≤200 mmHg, assessed by blood gas analysis performed with positive end-expiratory pressure (PEEP) maintained between 5 and 10 cmH2O.
ARDS survivors enrolled from five Italian intensive care units within 40 km of Milan were evaluated at 12 months. The follow-up trial consisted of physical examination, arterial blood gas analysis, high-resolution computed tomography (HRCT) of the lungs, pulmonary function test, standardized 6-min walking test evaluated with continuous oximetry [24], and assessment of health-related quality of life measured with the medical outcomes study 36-item form general health survey (SF-36) [25] and with the St. George’s Respiratory Questionnaire (SGRQ) [26]. Patients were invited to participate in the follow-up trial by telephone. Each patient was interviewed and examined in a single day at Ospedale Maggiore Policlinico. If the patient missed the follow-up trial, the patient could request another trial.
The study was approved by the institutional review board of the hospital. Written consent for the follow-up study was obtained directly from the patient at the time of the visit. Simplified Acute Physiology Score II [27] and Sequential Organ Failure Assessment (SOFA) [28] were recorded at study enrollment. Other patient characteristics are reported in Table 1. Assessors were blinded to treatment group.
Blood gas analysis
Arterial blood samples were obtained from the radial artery while the patient was sitting and breathing room air. Blood gas analysis was performed by using an automated blood gas measurement system (GEM® Premier™ 4000; Instrumentation Laboratory, Bedford, USA).
Pulmonary function studies
Pulmonary function tests were carried out as standard examination at a pulmonary function laboratory. Diffusion capacity was measured by the single-breath carbon-monoxide technique. All pulmonary function measurements were performed using a DLCO Spirometer (GANSHORN Medizin Electronic GmbH, Germany). Predicted values were generated according to CECA’s prediction equation [29].
Walking test data were analyzed using VISION SpO2 data management software.
Health-related quality of life
The SF-36 questionnaire consists of 36 validated items, covering eight general health aspects concerning physical functioning, role physical, role emotional, vitality, general health, bodily pain, social functioning, and mental health [25]. The eight health aspects are defined as follows: physical functioning, the extent to which health limits physical activity; physical role, the extent to which physical health interferes with work or limits activity; pain, the intensity of pain and the effect of pain on patient’s ability to work; general health, patient’s own evaluation of his or her health outlook; vitality, the degree of energy the patient has; social functioning, the extent to which health or emotional problems interfere with work or activities; and mental health, general health. From these items, summary scores for physical and mental health are calculated. Scores ranging from 0 to 100 were computed for the eight dimensions. Higher scores indicate better health-related quality of life [25]. A 5-point change in SF-36 has been determined to be a clinically meaningful difference [30].
The SGRQ is a pulmonary disease-specific health-related quality of life questionnaire covering three domain: symptoms, activity, and impact on daily life [26]. The symptoms domain relates to respiratory symptoms and their frequency and severity; the activity domain relates to activities that cause or are limited by breathlessness; the impact domain covers a range of aspects concerned with social functioning and psychological disturbances resulting from airway disease. Scores range from 0 to 100, and lower scores indicate better pulmonary-specific health-related quality of life [26].
Quantitative lung CT scan analysis
High-resolution CT scan (HRCT) of the lungs was performed in all patients in supine position with the following parameters: 1 mm sections at 15 mm intervals, 140 kV, 240 mA from the lung apices to the bases. Images were reconstructed with a high-spatial-resolution algorithm for parenchymal analysis. The outline of the lungs was manually drawn in each image, and cross-sectional lung images were processed and analyzed using a custom-designed software package, as previously discussed [31]. The volume of lung regions with different degree of aeration, classified as not aerated (density between +100 and −100 Hounsfield units), poorly aerated (−101 and −500 H units), normally aerated (density between −501 and −900 H units), and overinflated (density between −901 and −1,000 H units), were computed and expressed as percentages of total lung weight [32].
Statistical analysis
Patient characteristics, pulmonary function, and health-related quality of life data are reported as median, mean ± standard deviation (SD), and interquartile range (25th and 75th percentiles). Wilcoxon rank-sum test and unpaired Student’s t test were used for continuous variables and Fisher’s exact test for categorical variables. Differences were considered significant for p < 0.05. All statistical tests were performed with SAS(c) version 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
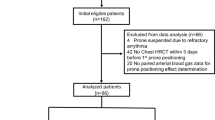
At the follow-up, 26 patients were evaluated. Median follow-up times were 12.8 (11–13) and 12.6 (11–13) months for the prone and supine group, respectively. The overall mortality rate at the follow-up was 60% (113 patients), of which 12% (14 patients) took place from 6 to 12 months. Mortality of the patients screened at 12 months was not different between the prone and supine group (9/38 versus 5/43, respectively, p = 0.24; Fig. 1).
Reasons for exclusion are outlined in Fig. 1. The patients evaluated did not have any baseline clinical differences compared with those not evaluated or compared with the total number of patients enrolled in the PSII study.
The descriptive baseline and hospital data for the evaluated patients are presented in Table 1. There were no significant differences in age, sex, initial illness severity (SAPS II score), or SOFA score between patients ventilated in supine versus prone position. There were no statistically differences in gas exchange or mechanical ventilation during the first week of treatment between the groups.
Days of mechanical ventilation and length of intensive care stay in the prone and supine group were similar at 13 (8–28) versus 16 (10–23) days and 29 (16–45) versus 16 (10–23) days (p = 0.22 and p = 0.15), respectively.
At the follow-up, the mortality rate was not different between the prone versus supine group (64.1% versus 56.8%, respectively). However, from inclusion to follow-up, there was a trend for higher mortality in the prone compared with the supine group. Regarding pulmonary function, lung volumes were normal since the median values were always higher than 80% of predicted values, at similar levels in both groups (Table 2).
Arterial oxygenation was slightly higher in the supine compared with the prone group (Table 2); however, arterial oxygenation corrected for patient age [33] was similar between the two groups (90.2 ± 5.0 versus 88.4 ± 4.2 mmHg). None of the patients were receiving supplementary oxygen. There was not any difference in arterial carbon dioxide or carbon monoxide diffusion capacity.
Distance walked in 6 min was similar between the two groups (Table 3), and in only one patient did arterial oxygenation fall below 90% during the 6-min walking test.
Table 3 shows the extension of parenchymal changes seen on HRCT. The mean amounts of not aerated, poorly aerated, and well-aerated lung regions were 8%, 16%, and 76% of total lung weight, being similar between the prone and supine group. The prone group had a higher amount of overaerated lung regions compared with the supine group. There was not any difference in the distribution of not or poorly aerated lung regions according to the sternal vertebral gradient.
Health-related quality of life is summarized in Table 4. The scores for all domains of SF-36 were similar to those of an age- and sex-matched control population. There were no significant differences between the prone and supine group in any of the SF-36 categories.
Unlike in the SF-36, in both groups the SGRQ showed a slight, but significant, impairment of quality of life and a reduction in the level of activity due to respiratory disease compared with the control population. However, there were no significant differences between the prone and supine group concerning SGRQ score.
Discussion
The current study showed that (1) at follow-up in evaluated ARDS patients, pulmonary function, gas exchange, and lung CT scan analysis were within normal values; (2) SF-36 score was similar to that of healthy subjects, while SGRQ showed impairment of daily activity due to pulmonary disease; (3) there were no differences in any of the tested variables (except for overinflated tissue) between patients treated in prone or supine position.
Mortality rate and pulmonary function
As regards mechanical management of ALI/ARDS patients, only application of a low-tidal-volume strategy has been shown to significantly decrease the mortality rate [34]. However, based on the available data, also prone position has been advocated as a rescue maneuver to improve patient outcome [20–22], especially in the most severe ARDS patients [19, 21–23], due to its capability to reduce overall stress–strain [15–18] and ventilator-induced lung injury [14].
The intensive care mortality rate in ALI/ARDS patients can range between 31% and 65% [3, 35], depending on the severity of lung injury, the number of failed nonpulmonary organs, and the patient’s underlying condition [36]. Moreover, ALI/ARDS patients can continue to present a higher mortality rate after intensive care discharge compared with non-ARDS mechanically ventilated patients [37, 38]. Angus et al. reported that mortality increased from 31% at 1 month to 45% at 6 months [5], while Herridge et al. showed an 11% mortality rate from discharge up to 1 year [4]. Similarly, in the present study, the mortality rate was 50% at 6 months and increased to 60% at 12 months. Unfortunately we do not know the causes of death during follow-up, thus we cannot explain whether this high mortality rate was primarily due to respiratory function impairment after acute respiratory failure or to nonpulmonary organ failure.
It was reported that impairment of pulmonary function, evaluated by spirometric test, can be a relatively common finding in surviving ARDS patients, being present in up to 70% of patients at 1 month [7, 39]. However, within the first year, pulmonary function, originally abnormal, can improve, but at 12 months, 20–50% of surviving ARDS patients can still present significant impairment in pulmonary function [4, 7–10] that usually does not ameliorate thereafter [39].
Contrary to these data, in our patients, pulmonary function was minimally impaired; median values of all spirometric tests were always higher than 80% of predicted values in the two groups.
Few studies have analyzed possible iatrogenic causes of persistence of abnormal lung function. Klein et al. [40], in a group of patients with ARDS due to trauma, did not find any association between duration of mechanical ventilation and impairment of lung function. Ghio et al. [7] found a relationship between increase in pulmonary artery pressures and impairment in pulmonary function. Our patients received mechanical ventilation following the recommendation, which may have prevented ventilator-induced lung injury [41, 42], while mean duration of mechanical ventilation was similar to in previous studies [4, 7].
In addition to impairment on spirometric testing, reduction in DLCO has been found in surviving ARDS patients, with frequency between 40% and 80% [4, 7, 9, 10, 39, 43]. This persistent impairment in gas transfer is probably due to injury at capillary level, which promotes thickening in alveolar capillary interfaces, pulmonary fibrosis, and pulmonary vascular remodeling. Similarly to the spirometric data, DLCO and gas exchange were within the normal range values and without any difference between the two groups.
Evaluation of ability to walk for a distance is an inexpensive measure of physical function and is reduced in several diseases such as heart failure, obstructive lung disease, and neuromuscular disease [44, 45]. We found that the distance walked in the 6-min walking test was 10% lower than the predicted value. These data are similar to those previously reported by Herridge et al. [4] in ARDS survivors. Unfortunately we do not know whether this reduction could be due to dyspnea or to muscle weakness.
High-resolution CT scan
It was reported in surviving ARDS patients evaluated for lung morphology by lung CT scan after a period of 6–20 months that the reticular pattern was present in up to 80% of these patients [9, 46–48]. However, the mean extension of this injury was less than 10–15% of total lung volume [9, 46–48]. This reticular pattern is usually found in nondependent lung regions, reflecting the highly traumatizing effect of mechanical ventilation, i.e., higher overdistension–overstress, compared with dependent lung regions, in which consolidated/collapsed areas [9, 46, 48] may protect the lung from injury by mechanical ventilation. The relationship between development of lung fibrosis after ARDS and any possible risk factor is not straightforward. Some studies have found significant correlation between CT scan impairment and duration of mechanical ventilation, level of PEEP, and oxygen fraction [49, 50]. However, these data may only reflect greater severity of ARDS, which can be responsible per se for lung fibrosis [46].
Compared with previous studies in which only morphological visual analysis was done, we performed quantitative lung analysis of CT scan images to quantify lung alterations. In this analysis, four lung compartments are usually defined: normally aerated, poorly aerated (which is equivalent to ground-glass and reticular pattern), not aerated (which corresponds to collapsed and/or consolidated areas), and hyperinflated (which reflects overfilled lung regions) [32]. Poorly aerated lung regions accounted for a mean of 15% of total lung weight, without any difference between prone and supine group or between vertebral–dorsal distribution. Conversely, overaerated lung regions were significantly more present in the prone group, although this difference was not associated with any functional effect.
Health-related quality of life
Despite a consensus statement in 1994 suggesting that future outcome evaluation in intensive care should incorporate assessment of quality of life [38], it was reported that less than 2% of all critical care studies evaluated HRQL [51]. The most commonly used scale for evaluating the HRQL is the Short Form-36 (SF-36) general health survey. SF-36 is a generic questionnaire which assesses eight items from physical functioning to mental health [52]. It was previously shown that surviving ARDS patients presented significantly lower SF-36 scores at 1 year compared with comparably ill or injured patients without ARDS [4, 5, 8–11]. This reduction in HRQL was related to alteration in pulmonary function [10], impairment in muscle function [4], and duration of mechanical ventilation [13]. However, some authors suggested assessing severity of illness in surviving ARDS patients by means of St. George’s Respiratory Questionnaire (SGRQ) [26]. A difference in total SGRQ score of about 4 points would indicate a clinically significant difference between populations [26]. SGRQ is a specific pulmonary disease questionnaire validated in airway disease [26]. Although the SGRQ has never been validated in ARDS patients, in whom dyspnea may also be related to extrapulmonary diseases and the relationship between laboratory measurements and symptoms might be very complex, significant alteration in all SGRQ domains in surviving ARDS patients has been reported to different extents [8, 9, 37], with older patients presenting significantly greater impairments [9]. In this study, SF-36 scores were within the normal range while SGRQ reported significant reduction in domain scores that evaluated activity (extent to which symptoms limit daily activity) and impact of the disease on patients’ social and emotional lives [53]. Thus, although our patients presented normal pulmonary function and SF-36 scores, they reported pulmonary symptoms that reduced daily activities, suggesting that the patients’ subjective perception of symptoms is not only related to physiological measurements [54, 55].
Limitations
Possible limitations of the current study are the small number of patients enrolled (39%, 26/67) compared with the eligible patients and the absence of any data regarding the number of patients who received rehabilitation before the follow-up. Although the evaluated patients did not have any baseline clinical differences compared with the nonevaluated patients (i.e., those lost to follow-up) or compared with the total sample of enrolled patients in the prone–supine study (PSII), we cannot exclude the possibility of substantial selection bias.
Conclusions
The present study showed that the mortality rate at 1 year after ARDS was high. However, in this small group of ARDS survivors, lung CT scan and pulmonary function were not different in patients treated in prone versus supine position.
References
Ashbaugh DG, Bigelow DB, Petty TL, Levine BE (1967) Acute respiratory distress in adults. Lancet 2:319–323
Gattinoni L, Carlesso E, Cadringher P, Valenza F, Vagginelli F, Chiumello D (2003) Physical and biological triggers of ventilator-induced lung injury and its prevention. Eur Respir J Suppl 47:15s–25s
Ferguson ND, Frutos-Vivar F, Esteban A, Anzueto A, Alia I, Brower RG, Stewart TE, Apezteguia C, Gonzalez M, Soto L, Abroug F, Brochard L (2005) Airway pressures, tidal volumes, and mortality in patients with acute respiratory distress syndrome. Crit Care Med 33:21–30
Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS (2003) One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 348:683–693
Angus DC, Musthafa AA, Clermont G, Griffin MF, Linde-Zwirble WT, Dremsizov TT, Pinsky MR (2001) Quality-adjusted survival in the first year after the acute respiratory distress syndrome. Am J Respir Crit Care Med 163:1389–1394
Cheung AM, Tansey CM, Tomlinson G, Diaz-Granados N, Matte A, Barr A, Mehta S, Mazer CD, Guest CB, Stewart TE, Al Saidi F, Cooper AB, Cook D, Slutsky AS, Herridge MS (2006) Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med 174:538–544
Ghio AJ, Elliott CG, Crapo RO, Berlin SL, Jensen RL (1989) Impairment after adult respiratory distress syndrome. An evaluation based on American Thoracic Society recommendations. Am Rev Respir Dis 139:1158–1162
Heyland DK, Groll D, Caeser M (2005) Survivors of acute respiratory distress syndrome: relationship between pulmonary dysfunction and long-term health-related quality of life. Crit Care Med 33:1549–1556
Linden VB, Lidegran MK, Frisen G, Dahlgren P, Frenckner BP, Larsen F (2009) ECMO in ARDS: a long-term follow-up study regarding pulmonary morphology and function and health-related quality of life. Acta Anaesthesiol Scand 53:489–495
Orme J Jr, Romney JS, Hopkins RO, Pope D, Chan KJ, Thomsen G, Crapo RO, Weaver LK (2003) Pulmonary function and health-related quality of life in survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med 167:690–694
Davidson TA, Caldwell ES, Curtis JR, Hudson LD, Steinberg KP (1999) Reduced quality of life in survivors of acute respiratory distress syndrome compared with critically ill control patients. JAMA 281:354–360
Dowdy DW, Eid MP, Dennison CR, Mendez-Tellez PA, Herridge MS, Guallar E, Pronovost PJ, Needham DM (2006) Quality of life after acute respiratory distress syndrome: a meta-analysis. Intensive Care Med 32:1115–1124
Schelling G, Stoll C, Vogelmeier C, Hummel T, Behr J, Kapfhammer HP, Rothenhausler HB, Haller M, Durst K, Krauseneck T, Briegel J (2000) Pulmonary function and health-related quality of life in a sample of long-term survivors of the acute respiratory distress syndrome. Intensive Care Med 26:1304–1311
Gattinoni L, Vagginelli F, Carlesso E, Taccone P, Conte V, Chiumello D, Valenza F, Caironi P, Pesenti A (2003) Decrease in PaCO2 with prone position is predictive of improved outcome in acute respiratory distress syndrome. Crit Care Med 31:2727–2733
Valenza F, Guglielmi M, Maffioletti M, Tedesco C, Maccagni P, Fossali T, Aletti G, Porro GA, Irace M, Carlesso E, Carboni N, Lazzerini M, Gattinoni L (2005) Prone position delays the progression of ventilator-induced lung injury in rats: does lung strain distribution play a role? Crit Care Med 33:361–367
Mentzelopoulos SD, Roussos C, Zakynthinos SG (2005) Prone position reduces lung stress and strain in severe acute respiratory distress syndrome. Eur Respir J 25:534–544
Galiatsou E, Kostanti E, Svarna E, Kitsakos A, Koulouras V, Efremidis SC, Nakos G (2006) Prone position augments recruitment and prevents alveolar overinflation in acute lung injury. Am J Respir Crit Care Med 174:187–197
Broccard AF, Shapiro RS, Schmitz LL, Ravenscraft SA, Marini JJ (1997) Influence of prone position on the extent and distribution of lung injury in a high tidal volume oleic acid model of acute respiratory distress syndrome. Crit Care Med 25:16–27
Gattinoni L, Tognoni G, Pesenti A, Taccone P, Mascheroni D, Labarta V, Malacrida R, Di Giulio P, Fumagalli R, Pelosi P, Brazzi L, Latini R (2001) Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med 345:568–573
Guerin C, Gaillard S, Lemasson S, Ayzac L, Girard R, Beuret P, Palmier B, Le QV, Sirodot M, Rosselli S, Cadiergue V, Sainty JM, Barbe P, Combourieu E, Debatty D, Rouffineau J, Ezingeard E, Millet O, Guelon D, Rodriguez L, Martin O, Renault A, Sibille JP, Kaidomar M (2004) Effects of systematic prone positioning in hypoxemic acute respiratory failure: a randomized controlled trial. JAMA 292:2379–2387
Mancebo J, Fernandez R, Blanch L, Rialp G, Gordo F, Ferrer M, Rodriguez F, Garro P, Ricart P, Vallverdu I, Gich I, Castano J, Saura P, Dominguez G, Bonet A, Albert RK (2006) A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med 173:1233–1239
Taccone P, Pesenti A, Latini R, Polli F, Vagginelli F, Mietto C, Caspani L, Raimondi F, Bordone G, Iapichino G, Mancebo J, Guerin C, Ayzac L, Blanch L, Fumagalli R, Tognoni G, Gattinoni L (2009) Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA 302:1977–1984
Gattinoni L, Carlesso E, Taccone P, Polli F, Guerin C, Mancebo J (2010) Prone positioning improves survival in severe ARDS: a pathophysiologic review and individual patient meta-analysis. Minerva Anestesiol 76:448–454
Weisman IM, Zeballos RJ (2001) Clinical exercise testing. Clin Chest Med 22:679–701
McHorney CA, Ware JE Jr, Lu JF, Sherbourne CD (1994) The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care 32:40–66
Jones PW, Quirk FH, Baveystock CM (1991) The St. George’s Respiratory Questionnaire. Respir Med 85:25–31 Suppl B
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
Report working party (1983) Standardized lung function testing. Bull Eur Physiopathol Respir 19(Suppl 5):1–95
Ware JE, Snow KK, Kosinski M, Gandek B (1993) SF-36 health survey: manual and interpretation guide. Boston: Mass: The Health Institute Med Care 30:473–483
Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G (2006) Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 354:1775–1786
Gattinoni L, Caironi P, Pelosi P, Goodman LR (2001) What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med 164:1701–1711
Fallon MB, Abrams GA (2000) Pulmonary dysfunction in chronic liver disease. Hepatology 32:859–865
Brower RG, Morris A, MacIntyre N, Matthay MA, Hayden D, Thompson T, Clemmer T, Lanken PN, Schoenfeld D (2003) Effects of recruitment manoeuvres in patients with acute lung injury and acute respiratory distress syndrome ventilated with high positive end-expiratory pressure. Crit Care Med 31:2592–2597
The Acute Respiratory Distress Syndrome Network (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301-1308
Brun-Buisson C, Minelli C, Bertolini G, Brazzi L, Pimentel J, Lewandowski K, Bion J, Romand JA, Villar J, Thorsteinsson A, Damas P, Armaganidis A, Lemaire F (2004) Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med 30:51–61
Davidson TA, Rubenfeld GD, Caldwell ES, Hudson LD, Steinberg KP (1999) The effect of acute respiratory distress syndrome on long-term survival. Am J Respir Crit Care Med 160:1838–1842
(1994) Predicting outcome in ICU patients. 2nd European Consensus Conference in Intensive Care Medicine. Intensive Care Med 20:390-397
McHugh LG, Milberg JA, Whitcomb ME, Schoene RB, Maunder RJ, Hudson LD (1994) Recovery of function in survivors of the acute respiratory distress syndrome. Am J Respir Crit Care Med 150:90–94
Klein JJ, van Haeringen JR, Sluiter HJ, Holloway R, Peset R (1976) Pulmonary function after recovery from the adult respiratory distress syndrome. Chest 69:350–355
Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, Slutsky AS, Pullenayegum E, Zhou Q, Cook D, Brochard L, Richard JC, Lamontagne F, Bhatnagar N, Stewart TE, Guyatt G (2010) Higher versus lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 303:865–873
Cesana BM, Antonelli P, Chiumello D, Gattinoni L (2010) Positive end-expiratory pressure, prone positioning, and activated protein C: a critical review of meta-analyses. Minerva Anestesiol 76:929–936
Peters JI, Bell RC, Prihoda TJ, Harris G, Andrews C, Johanson WG (1989) Clinical determinants of abnormalities in pulmonary functions in survivors of the adult respiratory distress syndrome. Am Rev Respir Dis 139:1163–1168
Butland RJ, Pang J, Gross ER, Woodcock AA, Geddes DM (1982) Two-, six-, and 12-minute walking tests in respiratory disease. Br Med J (Clin Res Ed) 284:1607–1608
Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, Berman LB (1985) The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 132:919–923
Desai SR, Wells AU, Rubens MB, Evans TW, Hansell DM (1999) Acute respiratory distress syndrome: CT abnormalities at long-term follow-up. Radiology 210:29–35
Kim SJ, Oh BJ, Lee JS, Lim CM, Shim TS, Lee SD, Kim WS, Kim DS, Kim WD, Koh Y (2004) Recovery from lung injury in survivors of acute respiratory distress syndrome: difference between pulmonary and extrapulmonary subtypes. Intensive Care Med 30:1960–1963
Nobauer-Huhmann IM, Eibenberger K, Schaefer-Prokop C, Steltzer H, Schlick W, Strasser K, Fridrich P, Herold CJ (2001) Changes in lung parenchyma after acute respiratory distress syndrome (ARDS): assessment with high-resolution computed tomography. Eur Radiol 11:2436–2443
Collins JF, Smith JD, Coalson JJ, Johanson WG Jr (1984) Variability in lung collagen amounts after prolonged support of acute respiratory failure. Chest 85:641–646
Pratt PC, Vollmer RT, Shelburne JD, Crapo JD (1979) Pulmonary morphology in a multihospital collaborative extracorporeal membrane oxygenation project. I. Light microscopy. Am J Pathol 95:191–214
Heyland DK, Guyatt G, Cook DJ, Meade M, Juniper E, Cronin L, Gafni A (1998) Frequency and methodologic rigor of quality-of-life assessments in the critical care literature. Crit Care Med 26:591–598
Chrispin PS, Scotton H, Rogers J, Lloyd D, Ridley SA (1997) Short form 36 in the intensive care unit: assessment of acceptability, reliability and validity of the questionnaire. Anaesthesia 52:15–23
Apolone G, Mosconi P (1998) The Italian SF-36 Health Survey: translation, validation and norming. J Clin Epidemiol 51:1025–1036
Alonso J, Anto JM, Gonzalez M, Fiz JA, Izquierdo J, Morera J (1992) Measurement of general health status of non-oxygen-dependent chronic obstructive pulmonary disease patients. Med Care 30:MS125–MS135
Mahler DA, Matthay RA, Snyder PE, Wells CK, Loke J (1985) Sustained-release theophylline reduces dyspnea in nonreversible obstructive airway disease. Am Rev Respir Dis 131:22–25
Acknowledgments
KCI supports the secretarial activity of the coordinating center
Conflicts of interest
Dr Gattinoni has been a member of KCI Medical Products advisory board. The other authors reported no financial disclosures.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chiumello, D., Taccone, P., Berto, V. et al. Long-term outcomes in survivors of acute respiratory distress syndrome ventilated in supine or prone position. Intensive Care Med 38, 221–229 (2012). https://doi.org/10.1007/s00134-011-2445-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2445-4