Abstract
Purpose
In critically ill patients with acute respiratory failure (ARF), fiberoptic bronchoscopy and bronchoalveolar lavage (FOB-BAL) are important tools in diagnostic strategies. In nonintubated patients, the patient’s agitation may lead to desaturation and compromise the realization of FOB. The aim of this study was to assess the feasibility and safety of target-controlled (TCI) propofol sedation during FOB-BAL in nonintubated hypoxemic patients.
Methods
The first end point in our prospective investigation within an intensive care unit (ICU) was the avoidance of endotracheal intubation within 24 h. Secondary end points were changes in the PaO2/FiO2 ratio, hemodynamic stability, patient comfort, occurrence of adverse effects, and quality of FOB. Patients self-evaluated their comfort after FOB.
Results
Twenty-four FOBs were performed in 23 patients with ARF. PaO2/FiO2 before FOB was 181 ± 50 (range 85–286). All patients tolerated FOB with BAL. None was intubated during the 2 h after FOB. Loss of consciousness was obtained with an effect site concentration of propofol of 1.49 ± 0.46 μg/mL (range 2.6–0.6). No significant adverse events occurred. TCI propofol allowed us to obtain amnesia, patient comfort, and it did not impair airway protection. Any hemodynamic changes observed were modest and transient.
Conclusions
FOB-BAL, under NIV and TCI with propofol, is feasible and safe in nonintubated patients with ARF. The TCI of propofol during FOB-BAL reduces patient discomfort with no significant adverse effects.
Similar content being viewed by others
Introduction
Fiberoptic bronchoscopy (FOB) is an important tool for the diagnosis of pulmonary diseases, especially infectious pneumonia [1]. In hypoxemic patients, several authors have shown that performing FOB under noninvasive ventilation (NIV) can preserve oxygenation [2–5]. The recent French consensus on NIV recommends performing FOB under NIV in patients with acute hypoxemic respiratory failure [6]. Nevertheless, this procedure remains uncomfortable [7]. In addition, the patient’s agitation may lead to desaturation and compromise the realization of FOB [8].
A few studies have evaluated the use of sedation during FOB, but only in nonhypoxemic patients [7, 9–12]. In this population, by reducing agitation with sedation, FOB is better tolerated and improves its efficacy [7, 11, 13]. For outpatients, most guidelines [14, 15] recommend the use of sedation for bronchoscopy. To our knowledge, only one study has evaluated the beneficial effect of sedation during a FOB with bronchoalveolar lavage (BAL) in hypoxemic patients [16].
By virtue of the specific pharmacodynamic and pharmacokinetic parameters of propofol, the use of target-controlled infusion (TCI) is a relevant choice for this kind of short procedure [17, 18]. TCI is a modern way of administering anesthetics based on a pharmacokinetic protocol assisted by a computerized mathematical calculation of drug concentration. The ‘diprifusor’ TCI system (Diprifusor™, Fresenius Vial, Brezins, France) [19] has been developed as a standardized infusion system to administer propofol by TCI, and it is now widely used in clinical practice [17, 20]. TCI allows rapid and precise adjustment of propofol concentration according to the clinical response of the patient. Sedation with propofol in TCI preserves spontaneous ventilation and hemodynamic parameters [21–24]. We have previously reported the use of TCI with propofol to achieve adequate compliance with NIV [24].
Our hypothesis is that sedation by TCI with propofol is effective and safe in patients with acute hypoxemic respiratory failure who are undergoing FOB with BAL under NIV. Sedation could prevent desaturation and help toleration of FOB. We performed this study to assess the feasibility and safety of this approach before designing a randomized controlled study.
Methods
Inclusion criteria were adult patients with acute respiratory failure (ARF), as defined by clinical signs of respiratory failure (polypnea, use of accessory respiratory muscle), and a PaO2/FiO2 ratio less than 250 under NIV, who need FOB with BAL for a diagnosis. Exclusion criteria were contraindications for NIV [6]. Contraindications specific to the protocol were FOB with envisaged bronchial biopsies, acute coronary syndrome, thrombopenia less than 30 × 109/L despite a platelet transfusion, coagulation disorders, PaO2/FiO2 ratio less than 80 under NIV, persistent respiratory acidosis under NIV (pH less than 7.32), systolic blood pressure below 80 mmHg, propofol or xylocaine allergy, pregnancy, age less than 18 years or more than 90 years, and weight above 150 kg or below 30 kg.
The experimental protocol was approved by our local ethics committee. Written informed consent was obtained from each study participant or their next of kin. Patients could be included twice provided at least 24 h had elapsed between the two bronchoscopy procedures.
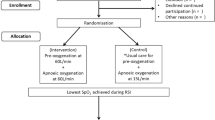
Between October 2007 and May 2008, all patients in our medical intensive care unit needing FOB for ARF were included. FOB was performed by an experienced respiratory physician. This used NIV and patients received sedation via TCI with propofol. The design of the procedure is shown in Fig. 1.
Design of procedure. Clinical parameters (respiratory rate, heart rate, and blood pressure) were recorded at the following times: before sedation (T0), during sedation but before bronchoscopy (T1), and 1 h after bronchoscopy (T2). Electrocardiography and pulse oximetry (SpO2) were continuously monitored throughout the procedure. Arterial blood samples were collected 15 min before and at 1 h after the FOB-BAL. Arterial blood gases under NIV. FOB-BAL fiberoptic bronchoscopy–bronchoalveolar lavage, NIV noninvasive ventilation, TCI target-controlled infusion
Topical anesthesia of the nose and larynx was performed with 5% lidocaine hydrochloride spray and 2% lidocaine hydrochloride, respectively.
Patients were placed under NIV before FOB to adapt to the ventilator and to settle the level of positive end-expiratory pressure (PEEP) and pressure support (PS). FiO2 was set to 100% during the procedure. Sedation with propofol, using TCI (Diprifusor™, Fresenius Vial, Brezins, France), was started prior to the FOB. The goal for defined sedation was an OAA/S level of 4 or 3 (response to verbal stimulation) [25] [Table 1 (ESM)]. The effect site concentration of propofol (Cpt) was initially set at 0.6 μg/mL and was increased by 0.2-μg/mL steps until the sedation goal was achieved. FOB was then performed. The fiberoptic bronchoscope (Olympus IT 30, Rungis, France) was inserted through the mask and then through the nose, as shown in Fig. 2. BAL was performed by wedging the bronchoscope into a subsegment of the area with the most marked X-ray abnormality. Sterile physiologic saline solution was infused (3 × 50-mL aliquots maximum), and gentle aspiration was performed after each infusion. BAL fluid (BALF) aliquots were pooled and immediately processed for cytological examination and quantitative bacterial cultures.
Patient undergoing FOB during NIV. FOB was performed by introducing the bronchoscope into the nose through the T-seal adapter and the full-face mask as shown in the picture. The mask had been secured with headstraps. The face mask was connected to a mechanical ventilator (not shown). We used a full-face mask (King Systems®) allowing us to match the nose to the T-seal adapter aperture
Sedation was stopped at the end of BAL. All patients were carefully monitored after FOB. NIV was maintained for at least 1 h after FOB, and FiO2 was gradually decreased until it returned to baseline values.
Criteria for endotracheal intubation
The predetermined criteria for endotracheal intubation (ETI) are failure to maintain a PaO2/FiO2 ratio greater than 80 under NIV, the development of conditions necessitating ETI to protect the airways (e.g., coma or seizure disorders), or to manage copious tracheal secretions, hemodynamic or ECG instability, or the inability to correct dyspnea.
Data collection
Simplified Acute Physiological II (SAPS II), demographic data, and underlying diseases were collected.
Clinical parameters (respiratory rate, heart rate, and blood pressure) were recorded under NIV just before sedation (T0), after the beginning of sedation just before bronchoscopy (T1), and at 1 h after bronchoscopy under NIV (T2). Electrocardiography, respiratory rate, and pulse oximetry (SpO2) were continuously monitored throughout the procedure. Arterial blood samples were collected under NIV at 15 min before and at 1 h after the FOB.
Volume of BAL aspiration, as a percentage of aliquot injection, was recorded. The quality of BAL was evaluated by cytological examination.
Any complications, such as hemoptysis, cardiac arrhythmias, cardiac arrest, over sedation, desaturation (desaturation was considered if, at any time, the patient’s SpO2 dropped below 90% for longer than 1 min, or even before these limits in cases of poor clinical tolerance), cough, agitation, pneumothorax, and the need for ETI within 24 h after the bronchoscopy, were recorded.
One hour after FOB, patients self-evaluated their comfort using a verbal analogous scale (VAS) that included four closed questions (Table 3).
A diagnostic impact of FOB-BAL was defined as identification of at least one the pathogens responsible for pulmonary infiltrates. A therapeutic impact of FOB-BAL was defined as the addition of a targeted treatment, discontinuation of a treatment, or narrowing the spectrum of antimicrobials.
End points
The first end point was the avoidance of endotracheal intubation within 24 h. Respiratory failure was attributed to FOB when ETI was needed during the 6 h after examination. The physicians involved in this study were not in charge of the patient studied and did not take part in their management. The clinical status of the patients was also evaluated at 24 h after FOB.
The secondary end points were changes in the PaO2/FiO2 ratio, the maintenance of hemodynamic stability, patient comfort, the occurrence of adverse effects, and the quality of FOB, as evaluated by cytological examination of the aliquots.
Statistical analyses
Statistical analyses were performed using XLstat 2008.2 (Addinsoft®). All baseline data were normally distributed (Shapiro–Wilk test). Clinical characteristics are reported as their means ± SDs. For nonsequential values, Student’s t test was used for continuous data to compare pre- and postsession parameters. A p value less than 0.05 was taken as statistically significant. The results are expressed as the mean ± 95% CI to improve the clinical significance when comparing pre- and postsession parameters. Nonparametric analysis of variance (Friedman test) and the Mann–Whitney U test were used for sequential values. Comfort was evaluated by expressing the responses of the different modalities as a percentage.
Results
Characteristics of the population before FOB
Between October 2007 and May 2008, 23 consecutives patients were included and 24 FOBs were performed. Patient mean age was 60 ±16 years (23–75). The patients’ characteristics are shown in Table 1. Reasons for bronchoscopy were the presence of new pulmonary infiltrates in immunocompromised patients (n = 20; 83.3%), suspected malignancy (n = 2; 8.33%), hospital-acquired pneumonia (n = 1; 4.16%), and acute diffuse infiltrative pneumonia (n = 1; 4.16%).
Twenty patients (83.3%) were receiving immunosuppressive therapies. Patients had AIDS (n = 4), renal transplantation (n = 1), bone marrow transplantation (n = 3), neutropenia (n = 10), and long-term corticosteroid treatment (n = 1). Patients could have several causes of immunosuppression. Five patients had a hematologic malignancy or a solid-tumor cancer, without neutropenia, at the time of FOB. Chronic heart (n = 5) or respiratory diseases (n = 6) were noted in 11 patients. All patients had acute hypoxemic respiratory failure and had NIV prior to the decision to perform bronchoscopy. The mean PaO2/FiO2 ratio before FOB was 181 ± 50 (85–286) under NIV.
Results
All patients had good tolerance of FOB-BAL. Physiological parameters over the study period are presented in Table 2.
No patient needed intubation during the 2 h after FOB. Only one patient (0.41%) required ETI during the 6 h after FOB (for deterioration in respiratory status). Three additional patients (12.5%) were intubated during the 24 h after FOB-BAL (one for deterioration in respiratory status, two for septic shock).
The PaO2/FiO2 ratio increased from 181 ± 50 (range 85–286) at baseline to 211 ± 103 (range 85–502) 1 h after the procedure (p = 0.95). The great majority of patients (66.6%) were not intubated during the ICU stay.
Loss of consciousness was obtained with a Cpt of 1.49 ± 0.46 μg/mL (range 2.6–0.6). At this low hypnotic concentration, no significant adverse events, including vomiting or apnea, were recorded. SpO2 was monitored constantly throughout the FOB and dropped below 90% for longer than 1 min in only one patient. In this case, the desaturation of oxygen was corrected rapidly by opening the airway by anterior displacement of the mandible and by reducing the level of sedation.
We observed a decrease in mean arterial blood pressure, heart rate, and respiratory rate during the procedure, but these hemodynamic changes were modest and transient (Table 2). Pressure support 11 ± 3 (range 16–6) and PEEP 6 ± 1 (range 8–4) were not changed during the FOB. The average duration of the FOB was 13.5 ± 5.5 (range 5–30). No cases of hemoptysis or pneumothorax were recorded within 24 h after the bronchoscopy.
The responses from the questionnaire on satisfaction (Table 3) show that sedation gave satisfactory comfort. It appears that sedation allows amnesia to be achieved.
Results of FOB
The procedure provided diagnostic or therapeutic information for 18 patients (75%). FOB-BAL led to diagnosis of bacterial infection (6), pneumocystis jirovecci pneumonia (5), Aspergillosis (1), Candida pneumonia (1), cytomegalovirus (CMV) pneumonia (1), human herpes virus 6 (HHV6) infection (3), carcinomatous lymphangitis (1), alveolar hemorrhage (5), or drug-induced hypersensitivity pneumonia in one patient. These results led to the introduction or discontinuation of treatment in 12 (50%) cases.
A mean of 44 ± 18 mL (range 80–15) of the 128 ± 34 mL (range 180–40) instilled saline solution was retrieved (34% yield). After cytological examination, no BAL was considered “superficial”.
Two patients underwent a bronchial biopsy during FOB for suspected carcinomatous lymphangitis.
Discussion
Despite the development of noninvasive diagnostic tests, FOB-BAL often remains necessary. As has been well underlined in the literature, mortality is higher when the cause of ARF remains undetermined. In an observational study, Azoulay et al. [1] showed that, in 148 patients admitted into 15 French ICUs over a 1-year period, FOB-BAL was the only conclusive investigation used to obtain diagnoses in 33.7% of these patients. A recent randomized trial concluded that, in hematology and oncology patients with hypoxemic ARF, FOB is safe when performed in the ICU and should be added to noninvasive tests if it is feasible early after ICU admission [26]. Thus, the focus should remain on ways to increase the safety, patient comfort, and improve results from this type of examination, and this was the aim of our study.
The tolerance of FOB-BAL in nonintubated patients with ARF has rarely been studied [16, 27]. Some studies, with few patients, have reported improved tolerance of FOB-BAL by use of continuous positive airway pressure (CPAP) [3, 28] or NIV via face mask [4, 5, 28] or helmet [2]. Azoulay et al. [1] found that deterioration in respiratory status occurred in 44.8% of patients who underwent FOB-BAL with high-flow oxygen, but in only 18.7% of patients who had FOB-BAL with NIV (p = 0.02). However, despite the recommendations [6], only a minority of FOBs are carried out under NIV: 16% in Azoulay et al. study [1] and 19% in an another recent study [26].
The patient’s agitation may lead to desaturation and can compromise the realization of FOB. In addition, this procedure remains uncomfortable. In a recent study, in patients with stable respiratory failure, Dreher et al. [16] performed FOB under sedation with midazolam, or midazolam and alfentanil. The major finding of this study was that alveolar hypoventilation, as estimated by an increase in transcutaneous PCO2 (PtcCO2), was substantial during FOB in both groups. In our study, we did not monitor PtcCO2, but the examination was performed under NIV and we did not use opiates. Moreover, we have previously demonstrated that using TCI of propofol during NIV reduces patient discomfort with no significant effect on respiratory function and improves acceptance of NIV [24]. Propofol is an anesthetic agent that can be administered in TCI to maintain a constant concentration in the target cerebral compartment (Cpt). TCI allows rapid and precise adjustment of propofol concentration according to the clinical response of the patient [17].
Our study confirms that TCI sedation with propofol preserves the patient’s spontaneous ventilation and does not alter hemodynamic parameters. The rapid recovery of patients after stopping propofol infusion makes it an attractive option, particularly in this type of short procedure. Moreover, propofol also has amnesic properties [24, 29]. Amnesia for painful or a very stressful experience is a very justifiable objective. Probably the good tolerance of FOB in our group of sedated patients can be explained by the amnesia (Table 3).
The very low concentration of propofol used (1.49 ± 0.46 μg/mL) permitted constant patient cooperation and did not compromise spontaneous respiration. Moreover, the use of sedation did not seem to impair airway protection. We noted the persistence of a cough reflex when the fiberoptic went past the vocal cords.
Only four patients (16.6%) needed ETI during the 24 h after bronchoscopy, and no ETI was performed within 2 h. This is concordant with recent results, showing that ETI was performed in 18.7% of a similar group of patients during the day following bronchoscopy [1]. FOB is usually associated with alterations in gas exchange. In hypoxemic intubated patients, FOB is often followed by a 30% drop in PaO2, with a return to baseline within 2 h [30]. We suggest that FOB may explain the deterioration in respiratory status within the first 2 h after the procedure, but participated only in the respiratory worsening after this time delay. Support for this hypothesis can be found in studies of ALI outcomes, in which the intubation rate is close to that seen in our study [31–33]. In a recent observational study relating the tolerance of 213 FOB to BAL procedures in nonintubated patients with ARF, Cracco et al. [27] showed that the factors associated with increased ventilatory support after FOB were similar to those associated with severe sepsis or community-acquired pneumonia (heart rate, respiratory rate, and blood urea concentration). This suggests that the deterioration in respiratory status might have been mainly related to the natural course of the disease, rather than to bronchoscopy. Thus, we can expect that some patients probably require increased ventilatory support, even without a bronchoscopy. The impact of FOB on the need to increase the ventilatory support is unclear. However, the use of sedation does not seem to increase the rate of ETI.
Using sedation during bronchoscopic procedures gives obvious benefits: comfort and amnesia for the patient, thus allowing the realization of a thorough examination as confirmed by the quality of the cytological examination of BALF. This procedure provided diagnostic or therapeutic information for 18 patients (75%): this is a high rate compared with the 63.4% in the study by Azoulay et al. [1]. This good diagnostic yield is probably due to the strict selection of our patients. Moreover, FOB was performed at ICU admission in 20 cases (80.8%), within 24 h after ICU admission in two cases (8.3%), and only later during the ICU stay in two cases (8.3%). We suppose that comfort brought about by the sedation is part of the success of this procedure. However, this finding needs to be objectified in a randomized study.
In our study, 34% of the instilled saline solution was retrieved vs. 40% in Azoulay et al.’s series [26]. These results are in line with Maitre et al.’s study [3] in which they reported that slightly, but significantly, less BALF was recovered in the CPAP group, although the total number of cells in this group tended to be larger. There too, the role of the sedation must be confirmed.
We report results of a pilot study without control group. Some limitations need to be outlined. First, the entire procedure must be performed with close patient monitoring. TCI and FOB must be performed by experienced physicians. Second, the Cpt of propofol is variable for each patient; thus, it is necessary to carry out a titration. Thirdly, during the FOB-BAL we did not have any data apart from SpO2 and scope monitoring. Fourthly, two unplanned transbronchial biopsies were done. Finally, the comfort evaluation was done very early after the end of the procedure.
In summary, FOB-BAL may have an important role in the diagnostic workup of selected critically ill patients, but should only be performed after diligent analysis of its risk and benefits. In high-risk patients, the tolerance of FOB-BAL can be altered by patient agitation or asynchronism with the ventilator. The present study shows that FOB with BAL, under NIV and using TCI with propofol, is feasible and safe in nonintubated patients with severe hypoxemia. Within the limits of this noncontrolled study, TCI with propofol seemed to reduce patient discomfort without causing any significant adverse effects, and it did not seem to increase the rate of ETI compared with values in the literature. Thus, we are now performing a multicentric, randomized, controlled study with the primary objective of determining if using TCI with propofol during FOB-BAL, under NIV, can substantially reduce deep oxygen desaturation and increase clinical tolerance of FOB (Clinical Trial.gov; NTC00741949).
References
Azoulay E, Mokart D, Rabbat A, Schlemmer B (2008) Diagnostic bronchoscopy in hematology and oncology patients with acute respiratory failure: prospective multicenter data. Crit Care Med 36:100–107
Antonelli M, Pennisi MA, Conti G, Bello G, Maggiore S, Michetti V, Cavaliere F, Meduri G (2003) Fiberoptic bronchoscopy during noninvasive positive pressure ventilation delivered by helmet. Intensive Care Med 29:126–129
Maitre B, Jaber S, Maggiore SM, Bergot E, Richard JC, Bakthiari H, Housset B, Boussignac G, Brochard L (2000) Continuous positive airway pressure during fiberoptic bronchoscopy in hypoxemic patients. Am J Crit Care Med 162(3 Pt 1):1063–1067
Antonelli M, Conti G, Rocco M, Arcangeli A, Cavaliere F, Proietti R, Meduri GU (2002) Noninvasive positive-pressure ventilation versus conventional oxygen supplementation in hypoxemic patients undergoing diagnostic bronchoscopy. Chest 121:1149–1154
Antonelli M, Conti G, Riccioni L, Meduri G (1996) Noninvasive positive pressure ventilation via face mask during bronchoscopy with BAL in high risk hypoxemic patients. Chest 110:724–728
(2006) Ventilation non invasive au cours de l’insuffisance respiratoire aiguë (nouveau-né exclu). In: 3ème conférence de consensus commune organisée par la SFAR, SPLF et la SRLF; Paris
Gonzalez R, De-la-Rosa-Ramirez I, Maldonado-Hernandez A, Dominguez-Cherit G (2003) Should by patients undergoing a bronchoscopy be sedated? Acta Anaesthesiol Scand 47:411–415
Clarkson K, Power C, O’Connell F, Burke C (1993) A comparative evaluation of propofol and midazolam as sedative agents in fiberoptic bronchoscopy. Chest 105:1029–1031
Putanati S, Ballerin J, Corbetta L (1999) Patient satisfaction with conscious sedation for bronchoscopy. Chest 115:1437–1440
Allen MB (1995) Sedation in fiberoptic bronchoscopy. BMJ 310:1333
Silvestri G, Vincent B, Wahidi M, Robinette E, Hansbrough J, Downie G (2009) A phase 3, randomized, double-blind study to assess the efficacy and safety of fospropofol disodium injection for moderate sedation in patients undergoing flexible bronchoscopy. Chest 135:41–47
Matot I, Kramer M (1997) Sedation in outpatient fiberoptic bronchoscopy: alfentanil-propofol vs meperidine-midazolam. Anesthesiology 87(Suppl 3):12A
Maguire GP, Rubinfeld AR, Trembath PW (1998) Patients prefer sedation for fiberoptic bronchoscopy. Respirology 3:81–85
Wood-Baker R, Burdon J, MacGregor A (2001) Fiber-optic bronchoscopy in adults: a position paper of The Thoracic Society of Australia New Zealand. Intern Med J 31:479–487
British Thoracic Society, Bronchoscopy Guidelines Committee (2001) British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax 56(Suppl):i1–i21
Dreher M, Ekkernkamp E, Storre JH, Kabitz H, Windisch W (2010) Sedation during flexible bronchoscopy in patients with pre-existing respiratory failure: midazolam versus midazolam plus alfentanil. Respiration 79:307–314
McMurray TJ, Johnston JR, Milligan KR, Grant IS, Mackenzie SJ, Janvier G, Glen JB (2004) Propofol sedation using Diprifusor™ target-controlled infusion in adult intensive care unit patients. Anaesthesia 59(7):636–641
Mazzarella B, Melloni C, Montanini S, Novelli GP, Peduto VA, Santandrea E, Vincenti E, Zattoni J (1999) Comparison of manual infusion of propofol and target controlled infusion: effectiveness, safety and acceptability. Minerva Anestesiol 65:701–709
Glen JB (1998) The development of ‘Diprifusor’: a TCI system for propofol. Anaesthesia 53:13–21
Casati A, Fanelli G, Casaletti E, Colnaghi E, Cedrati V, Torri G (1999) Clinical assessment of target-controlled infusion of propofol during monitored anesthesia care. Can J Anaesth 46:235–239
Nieuwenhuijs D, Sarton E, Teppema L, Dahan A (2000) Propofol for monitored anesthesia care: implications on hypoxic control of cardiorespiratory responses. Anesthesiology 92:46–54
Nieuwenhuijs D, Sarton E, Teppema L, Kruyt E, Olievier I, van Kleef J, Dahan A (2001) Respiratory sites of action of propofol: absence of depression of peripheral chemoreflex loop by low-dose propofol. Anesthesiology 95:889–895
Nagayova B, Dorirington E, Gilland W, Robbins A (1995) Comparaison of the effects of sub-hypnotic concentration of propofol and halothane on the acute ventilatory response to hypoxia. Br J Anaesth 75(6):713–718
Clouzeau B, Bui HN, Vargas F, Grenouillet-Delacre M, Guilhon E, Gruson D, Hilbert G (2010) Target-controlled infusion of propofol for sedation in patients with non-invasive ventilation failure due to low tolerance: a preliminary study. Intensive Care Med 36:1675–1680
Chernik DA, Gillings D, Laine H, Hendler J, Silver JM, Davidson AB, Schwam EM, Siegel JL (1990) Validity and reliability of the observer’s assessment of alertness/sedation scale: study with intravenous midazolam. J Clin Psychopharmacol 10:244–251
Azoulay E, Mokart D, Lambert J, Lemiale V, Chevret S, Schlemmer B (2010) Diagnostic strategy for hematology and oncology patients with acute respiratory failure. Am J Respir Crit Care Med 182:1038–1046
Cracco C, Fartoukh M, Prodanovic H, Azoulay E, Chenivesse C, Lorut C, Beduneau G, Bui HN, Taillé C, Brochard L, Demoule A, Maitre B (2008) Tolerance of fiberoptic bronchoscopy in patients with acute respiratory failure. Eur Respir J 32:768 S
Da Conceicao M, Genco G, Favier JC, Bidallier I, Pitti R (2000) Fiberoptic bronchoscopy during non invasive positive pressure ventilation in patients with chronic lung disease with hypoxemia and hypercapnia. Ann Fr Anesth Reanim 19:231–236
Skipsey IG, Colvin JR, Mackenzie N, Kenny GN (1993) Sedation with propofol during surgery under local blockade: assessment of a target-controlled infusion system. Anaesthesia 48:210–213
Trouillet JL, Guiguet M, Gibert C, Fagon JY, Dreyfuss D, Blanchet F, Chastre J (1990) Fiberoptic bronchoscopy in ventilated patients: evaluation of cardiopulmonary risk under midazolam sedation. Chest 97:927–933
Confalonieri M, Potena A, Carbone G (1999) Acute respiratory failure in patients with severe community-acquired pneumonia: a prospective randomized evaluation of noninvasive ventilation. Am J Respir Crit Care Med 160:1585–1591
Antonelli M, Conti G, Moro ML, Esquinas A, Gonzales-Diaz G, Confalonieri M, Passariello M, Meduri G (2001) Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: a multi-center study. Intensive Care Med 27:1718–1728
Delclaux C, L’Her E, Alberti C, Mancebo J, Abourg F, Conti G, Guérin C, Shortgen F, Lefort Y, Antonelli M, Lepage E, Lemaire F, Brochard L (2000) Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: a randomized controlled trial. JAMA 284:2352–2360
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Clouzeau, B., Bui, HN., Guilhon, E. et al. Fiberoptic bronchoscopy under noninvasive ventilation and propofol target-controlled infusion in hypoxemic patients. Intensive Care Med 37, 1969–1975 (2011). https://doi.org/10.1007/s00134-011-2375-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2375-1