Abstract
Purpose
Septic shock induces a decrease in dendritic cells (DCs) that may contribute to sepsis-induced immunosuppression. We analyzed the time course of circulating DCs in patients with septic shock and its relation to susceptibility to intensive care unit (ICU)-acquired infections.
Methods
We enrolled adult patients with septic shock (n = 43), non-septic shock (n = 29), and with sepsis without organ dysfunction (n = 16). Healthy controls (n = 16) served as reference. Blood samples were drawn on the day of shock (day 1), then after 3 and 7 days. Myeloid (mDC) and plasmacytoid (pDC) DCs were counted by flow cytometry. Cell surface HLA-DR expression was analyzed in both DC subsets.
Results
At day 1, median mDC and pDC counts were dramatically lower in septic shock patients as compared to healthy controls (respectively, 835 mDCs and 178 pDCs/ml vs. 19,342 mDCs and 6,169 pDCs/ml; P < 0.0001) but also to non-septic shock and sepsis patients (P < 0.0001). HLA-DR expression was decreased in both mDCs and pDCS within the septic shock group as compared to healthy controls. DC depletion was sustained for at least 7 days in septic shock patients. Among them, 10/43 developed ICU-acquired infections after a median of 9 [7.5–11] days. At day 7, mDC counts increased in patients devoid of secondary infections, whereas they remained low in those who subsequently developed ICU-acquired infections.
Conclusion
Septic shock is associated with profound and sustained depletion of circulating DCs. The persistence of low mDC counts is associated with the development of ICU-acquired infections, suggesting that DC depletion is a functional feature of sepsis-induced immunosuppression.
Similar content being viewed by others
Introduction
The immune response to microbial infection relies on both innate and adaptive components in a sequential and coordinated manner [1]. Dendritic cells (DCs) constitute a pivotal link between those two arms of the host response and play an important role in the integration of the inflammatory response. As “professional” antigen-presenting cells, they initiate and polarize the adaptive immune response in activating T cells. Cytokines released by DCs orient the phenotype of T cells and drive them into different T-helper (Th) patterns [2]. Whereas the initial pro-inflammatory response is characterized by a Th-1 pattern, a second phase often follows septic shock (SS) and damage of host tissue [3] and leads to a post-infective immunosuppressive state, which is characterized by a shift to a predominantly Th-2 response with decreased production of pro-inflammatory cytokines [4] and apoptosis of B- and T-lymphocytes [5]. Selective depletion of DCs in secondary lymphoid organs reported in murine models of sepsis [6, 7] and in patients who died from sepsis [8], points to an important role of DCs in the pathophysiology of sepsis-induced immune dysfunction.
Circulating DC subsets represent only a small subset of DCs compared to tissular DCs. Circulating DCs correspond mostly to DC precursors and to a few mature DCs re-circulating from lymph nodes. Conversely to interstitial DCs, which migrate in lymph nodes upon inflammation stimuli, little is known about the migrating capacity of circulating DCs. Indeed, they may interact with white blood cells before any migration step and could be directly recruited to the lymph nodes of injured tissues [9]. The DCs can be separated into myeloid (mDCs) and plasmacytoid (pDCs) according to the expression of the membrane markers CD11c and CD123 [10]. Antigen presentation is the primary function of mDCs, the “classical” DCs. pDCs express lower levels of type II HLA and release large amounts of type I interferons [11]. As circulating DCs cannot be counted by an automated hemocytometer, they have not been extensively studied during SS. Recently, an early decrease in circulating DCs has been reported during human SS [12]. Whether shock or infection by itself is associated with decreased circulating DCs is unclear. Finally, the influence of circulating DC counts on the risk of secondary infections in patients with SS is unknown. To address these questions, we used a flow cytometry-based assay to quantify circulating DCs and their HLA-DR surface expression at the time of diagnosis and during the first week of evolution in patients with SS using patients with sepsis or non-septic shock (NSS), and healthy people as controls.
Patients and methods
Patients
All consecutive patients with septic or NSS admitted to our tertiary ICU between August 2007 and May 2008 were eligible. Patients with sepsis were identified in the emergency department. Sepsis (S) was defined as the presence of two criteria for systemic inflammatory response syndrome (SIRS) without organ failure and SS as sepsis with the need for vasopressor despite fluid expansion [13, 14]. For patients with NSS, cardiogenic shock was defined by the presence of myocardial dysfunction (left ventricular ejection fraction less than 30% assessed by echocardiography) and the need for inotropic support by dobutamine (more than 5 μg/kg/min) or epinephrine at any dose in the absence of patent infection [15]. Hemorrhagic shock was defined as hypotension with acute hemorrhage requiring transfusion of at least four packs of red blood cells in 24 h. Exclusion criteria were age less than 18 years, pregnancy, do-not-resuscitate orders on admission, hematologic malignancy, immunodeficiency defined as HIV infection, presence of bone marrow or solid organ transplantation, daily corticosteroid therapy greater than 0.5 mg/kg prednisone equivalent, chemotherapy or any other immunosuppressive treatments. As DC counts vary with age, we enrolled healthy volunteers and elderly patients scheduled for elective cataract surgery to build a cohort of 16 age- and sex-matched healthy controls. Subjects were excluded from the control cohort if they had any inflammatory disease, received oral corticotherapy, or if they presented infectious symptoms the previous month. Our institution’s ethics committee approved the study. As it was purely observational and did not require any additional blood sampling as compared to routine care, consent was waived for ICU patients. Written informed consent was obtained for controls.
Data collection
We recorded demographic data, SAPS II and SOFA score on admission, and collected daily SOFA scores, duration of hemodynamic support, and ICU survival. All physiological variables obtained during routine patient monitoring were recorded using the Centricity Clinisoft® software (GE Healthcare). Patients were screened daily for the presence of ICU-acquired infection, using usual criteria for catheter-related, spontaneous bacteremia, and ventilator-associated pneumonia [16, 17]. Physicians in charge of the patients were blinded to the results of DC counts.
DCs analysis
Two-hundred microliters of blood sample was drawn for DC analysis. In ICU patients, the first sample was obtained on the morning following admission (day 1) and analysis was repeated at days 3 and 7. Quantification of DCs was performed with the Trucount® assay (BD Biosciences) as described elsewhere [18, 19]. Briefly, 100 μl of whole blood was incubated with a fixed number of beads and with a mixture of fluorescent antibodies (anti-CD45-APC-Cy7, anti-lineage-FITC, anti-HLA-DR-PerCP, anti-CD11c-PE, and anti-CD123-APC) or with corresponding isotypic controls. Cell analysis was performed on a FACSCanto cytometer (BD Biosciences) and at least 105 CD45+ cells were acquired. Human pDCs and mDCs were defined respectively, as CD123+HLA-DR+Lin− and CD11c+HLA-DR+Lin− cells. DC counts (cells per milliliter) were determined as: (cell number in gate “pDC” (or “mDC”)/bead number in the gate “beads”) × (bead number in Trucount tube/blood volume). Mean fluorescence index (MFI) for HLA-DR was analyzed on DCs subsets. Dot plots typical for a healthy control or patient with SS are shown in Fig. S1 in the supplementary material.
Statistical analysis
Categorical data are presented as number (%) and compared by using the chi square or Fisher’s exact tests as appropriate. Given the non-normal distribution of continuous variables (age, DC, leukocytes and lymphocytes counts, MFI), they are presented as median (interquartile range—IQR) and compared using the Mann–Whitney test. Comparisons of DC counts between the four patients’ groups were done using a Kruskal–Wallis test. Comparisons of paired variables between days 1, 3, and 7 were analyzed using a Friedman test. Results are shown as box plots with outliers shown as circles. The ratio day 7 DC count/day 1 DC count was categorized as greater or less than 1. Simple logistic regression was used to calculate the odds ratio of a value lower than 1 (i.e., decrease of DCs count) to predict ICU-acquired infections. To evaluate the value of mDC and pDC counts to predict the advent of ICU-acquired infections, receiver operating characteristic (ROC) curves were constructed. All analyses were done with SPSS 12.0 software.
Results
Population characteristics
Between August 2007 and May 2008, 88 patients were enrolled in the study. Patients’ characteristics are shown in Table 1. Patients with septic and NSS were comparable in terms of age, sex ratio, SAPS II, and SOFA score on admission. As expected, patients with sepsis had lower SAPS II and SOFA scores. ICU survival was similar between SS and NSS patients (62.7 and 62.1%, respectively). Septic shock patients had a lower lymphocyte count and a significantly longer ICU length of stay. Nine of these patients were bacteremic and microbiological documentation could not be obtained in five other patients (11.6%). None of the patients with sepsis had bacteremia and none developed SS.
Circulating DC counts are dramatically decreased during septic shock
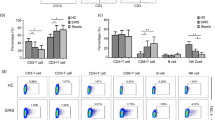
As shown in Fig. 1, mDC and pDC counts significantly differed between groups (P < 0.001 for both mDCs and pDCs). Patients with SS had lower mDC and pDC counts than healthy controls (P < 0.0001), NSS patients (P < 0.0001), and patients with sepsis (P < 0.0001). Note that patients with NSS also had lower circulating DC counts than healthy controls (P = 0.009 and P < 0.0001, respectively), whereas sepsis patients had lower circulating pDC counts (P = 0.02) but comparable mDC counts compared with healthy controls. Cell surface expression of HLA-DR was significantly lower on circulating mDCs and pDCs in SS patients, as compared to healthy controls (P = 0.005, and P = 0.037, respectively) (Fig. 2). Cell surface expression of HLA-DR on mDCs was significantly lower in NSS and sepsis patients than in healthy controls (P = 0.015 and P = 0.026, respectively), whereas no difference was observed on pDCs. Cell surface expression of HLA-DR on mDCs and pDCs was not different between SS, NSS, and sepsis patients. For all patients groups, we found no correlation between circulating DC counts and SAPS II, admission SOFA scores, or mortality rates (data not shown).
Comparison of circulating mDC and pDC counts in patients with septic shock (SS, grey boxes, N = 43), non-septic shock (NSS, dashed boxes, N = 29), sepsis (S, dotted boxes, N = 16), and healthy control (HC, white boxes, N = 16) at day 1. *mDC and pDC counts of NSS patients were lower than HC (P = 0.009 and P < 0.0001, respectively). §pDC count of S patients was lower than HC (P = 0.02). Comparisons between each group of patients are done using Mann–Whitney test
Comparison of HLA-DR expression on mDCs and pDCs in patients with septic shock (SS, grey boxes, N = 43), non-septic shock (NSS, dashed boxes, N = 29), sepsis (S, dotted boxes, N = 16), and healthy control (HC, white boxes, N = 16) at day 1; 8 and 3 patients of the SS and NSS groups have a pDC count less than 50/ml, so that MFI of HLA-DR could not be analyzed. Comparisons between each group of patients are done using Mann–Whitney test
Counts of circulating DC were measured at days 3 and 7 for patients with SS and NSS (Fig. 3). In patients with SS, mDC and pDC counts remained significantly lower than in NSS patients at day 3 (P = 0.013 and 0.025, respectively). A similar trend was observed at day 7. Expression of HLA-DR decreased significantly between day 1 and day 7 in both groups of patients (Fig. S2, supplementary material).
Evolution at days 1, 3, and 7 (D1, D3, D7) of circulating mDC and pDC counts in patients with septic shock (grey boxes, N = 40 at day 3 and 23 at day 7) or non-septic shock (dashed boxes, N = 17 at day 3 and 8 at day 7). Comparisons are done using Mann–Whitney test between SS and NSS patients day by day. *P < 0.0001, **P = 0.013, § P = 0.025
Persisting low mDC counts at day 7 is associated with ICU-acquired infections in SS patients
Among the 43 patients with SS, 10 presented 20 episodes of ICU-acquired infections (13 ventilator-associated pneumonia, 4 catheter-related infections, 2 peritonitis, and 1 mediastinitis) detailed in Table S1 in the online supplementary material. Table 2 summarizes the characteristics of those patients. The first episode occurred 9 [7.5–11] days following ICU admission. Figure 4 shows the time course of mDCs and pDCs in patients with or without ICU-acquired infection. Whereas mDC counts increased in patients without ICU-acquired infection (P = 0.023), mDCs remained low in those who developed at least one episode of infection. At day 7, mDC counts were significantly lower in patients who developed ICU-acquired infection (786/ml [101–2,345] vs. 2,878/ml [1,620–8,383] in patients without ICU-acquired infection P = 0.036). As shown in Fig. 5a, the variation in circulating mDC counts between days 1 and 7 (DC count day 7/DC count day 1) was lower in patients who developed ICU-acquired infections (0.44 [0.22–0.54] vs. 4.31 [1.53–7.54], P = 0.036). Thus, a decrease in mDC count between day 1 and day 7 was associated with an increased risk of subsequent infection (OR 22 [2.53–191], P = 0.005) with a predictive positive value of 80% and a negative predictive value of 84.6%. As shown in Fig. 5b, the day7/day1 ratio of mDCs has an area under the ROC curve (AUC) of 0.762 (CI 0.544–0.979) to predict the advent of an ICU-acquired infection. Despite a similar trend, circulating pDC counts did not significantly differ between patients with or without ICU-acquired infection (Fig 5a, b). Neither mDC HLA-DR expression at days 1, 3, or 7 nor its relative change were associated with ICU-acquired infection (data not shown). Changes in DCs count and HLA-DR expression for individual SS patients with or without ICU-acquired infections are shown in the online supplementary material (Figs. S4 and S5, respectively). In the NSS group, 4 patients developed ICU-acquired infections. The evolution of their DC counts is shown in Fig. S3 in the online supplementary material.
Evolution of mDCs and pDCs in septic shock patients with (grey boxes, N = 10) or without (white boxes, N = 33 at day 1, 30 at day 3, and 13 at day 7) ICU-acquired infection (IAI) at days 1, 3, and 7 (D1, D3 D7). *Comparison of repeated mDC counts in patients without ICU-acquired infection using Friedman test, P = 0.023. §Comparison of day 7 mDCs count between patients with and without ICU-acquired infection using Mann–Whitney test, P = 0.036
a Comparison of variation (i.e., ratio of day 7 and day 1 cell count) of mDCs and pDCs in patients with (grey boxes, N = 10) or without (white boxes, N = 13) ICU-acquired infection by Mann–Whitney test. b ROC curve of mDCs (black line) and pDCs (dashed line) ratio to assess ICU-acquired infection (IAI)
Discussion
Our study shows that, as compared to moderately severe infections and other types of shock, SS is associated with a profound decrease in circulating mDCs and pDCs. Moreover, a persistent decrease in mDCs is associated with the advent of ICU-acquired infection, suggesting the important role of these cells in post-infective immunodysfunction.
Very few studies investigated changes in circulating DCs in patients with SS [12, 20]. Guisset et al. [12] reported a decrease in initial DC counts that correlated with severity scores and survival. Interestingly, mortality rate reported in this study was higher (52.4%) than in our cohort despite lower SAPS II scores, indicating that factors not captured by the SAPS II score might have contributed to outcome. A case-mix of more severe SS patients with lower DC counts in our cohort might explain the absence of relationship between survival and DC counts. Poehlmann et al. [20] investigated changes in monocytes and DC counts on admission and 28 days after the onset of severe sepsis or SS. They also report a significant decrease in DC counts as compared to healthy controls, as well as decreased HLA-DR expression consistent with our results. A direct downregulation of HLA-DR expression at the surface of DCs or the differentiation of monocytes with sepsis-induced loss of HLA-DR expression [15, 21] into a subpopulation of circulating DCs with altered maturation could explain this finding. A decrease of membrane HLA-DR expression on DCs may also indicate DC apoptosis [22]. Finally, in the small cohort reported by Poehlmann et al. [20], DC counts failed to return to normal values in the 12 patients that were still alive at day 28, but the data provided do not permit any conclusion to be drawn regarding the link with ICU-acquired infection or patient outcome.
To our knowledge, our study is the first to demonstrate that circulating DCs are selectively depleted in SS as compared to shock from other etiologies and moderately severe infection. Our results indicate that the effects of infection and shock on DC counts were synergistic, as mDCs from patients with SS were decreased more than 20 times in comparison with healthy controls, whereas this factor was only about three for mDCs from patients with NSS. The mechanisms that may lead to a more profound depletion of DCs in the combined presence of infection and shock are unknown. One can speculate that activation of the inflammatory response through detection of pathogen-associated molecular patterns (PAMPs) and release of danger-associated molecular patterns [23] lead synergistically to profound DC depletion, whereas signals induced by recognition of PAMPs only have a modest effect on DC counts. Consistent with this hypothesis, a decrease in circulating DCs has been reported in infected burn patients, a clinical context in which infection and tissue damage coexist [24]. Alternatively, the loss of compartmentalization of the infection and the presence of overwhelming inflammation in the SS setting may contribute to activate molecular mechanisms that lead to the depletion of circulating DCs [25].
In our study, persistent depletion of mDCs is associated with the advent of ICU-acquired infection, highlighting the role of DCs in sepsis-induced immunosuppression. We have studied two subsets of circulating DCs and found only depletion of mDCs to be associated with secondary infection. As precursors of interstitial DCs, mDCs are the professional antigen-presenting cells and they contribute to initiate the adaptive immune response [26]. Together with monocytes and granulocytes they also secrete IL-12 as well as IL-10 [27]. The specific role of mDCs in antigen presentation as compared with pDCs suggests that the susceptibility to secondary infection involves an impairment of adaptive immunity. Several experimental studies suggest that quantitative and functional abnormalities of DCs increase susceptibility to secondary infection [28–30]. Indeed, polymicrobial sepsis induces persistent functional abnormalities of DCs characterized by impaired maturation, defective activation in response to Toll-like receptors agonists, imbalance between the production of IL-12 and IL-10 thereby skewing the immune response toward a Th-2 pattern, and a defective priming of T cell lymphocytes [30, 31]. Such functional abnormalities have been linked to increased susceptibility to secondary Pseudomonas aeruginosa pneumonia or invasive aspergillosis [28–30]. In humans, few data establish a link between sepsis-induced immunodysfunction and increased susceptibility to nosocomial sepsis. Besides quantitative disorders, circulating DCs from septic patients exhibit functional defects through a marked and prolonged impairment in pro-inflammatory cytokines production [20]. Most importantly, reduced monocyte HLA-DR expression, another feature of sepsis-induced immunodysfunction associated with impaired antigen presentation, has been associated with increased mortality [15, 21], and very recently to increased susceptibility to ICU-acquired infection [32]. Consistent with the data from Landelle et al. [32], our study suggests that sepsis-induced immune dysfunction might facilitate the development of nosocomial infections in humans.
Beside its monocentric design, our study has several limits. First, we did not precisely address the mechanism that leads to depletion of circulating DCs. We can reasonably hypothesize that apoptosis accounts for DCs depletion as previously demonstrated for depletion of spleen DCs in humans [8] and in murine models [6, 7, 33]. However, other mechanisms such as bone marrow insufficiency [34] or increased migration in peripheral tissues cannot be ruled out. Little is known regarding migration of circulating DCs in sepsis. Potential destinations of blood-borne DCs include the skin, lymph nodes, thymus, and spleen [9]. Although migration of circulating DCs to the spleen and lymph nodes might happen during SS, several studies have documented apoptosis-related depletion of DCs in these secondary lymphoid tissues in animal models of sepsis [6, 7, 33] suggesting that apoptosis is one the primary mechanisms leading to immune cells depletion. Second, despite the relatively large number of patients enrolled, the number of episodes was too low to perform a reliable multivariate analysis of factors associated with the risk of ICU-acquired infections. Finally, our data do not allow one to formally state whether DC depletion was a cause or a consequence of secondary infections. However, the fact that 80% of secondary infections developed after day 7 suggests that low mDC counts preceded the development of secondary infections. Greater availability of flow cytometry-based assays to get better insights into the immune status of patients during the clinical course of SS should allow one to confirm these findings in a larger cohort.
In summary, our data show that SS is specifically associated with a profound depletion of circulating DCs, associated with a downregulation of HLA-DR membrane expression. Persistent depletion of mDCs at day 7 is associated with the advent of ICU-acquired infection. Immuno-monitoring using flow cytometry-based assays may allow one to detect a subgroup of patients at high risk of secondary infections that might benefit from strategies designed to improve survival and restore the function of DCs.
References
Rittirsch D, Flierl MA, Ward PA (2008) Harmful molecular mechanisms in sepsis. Nat Rev Immunol 8:776–787
Shortman K, Liu YJ (2002) Mouse and human dendritic cell subtypes. Nat Rev Immunol 2:151–161
Annane D, Bellissant E, Cavaillon JM (2005) Septic shock. Lancet 365:63–78
Ertel W, Kremer JP, Kenney J, Steckholzer U, Jarrar D, Trentz O, Schildberg FW (1995) Downregulation of proinflammatory cytokine release in whole blood from septic patients. Blood 85:1341–1347
Hotchkiss RS, Nicholson DW (2006) Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol 6:813–822
Efron PA, Martins A, Minnich D, Tinsley K, Ungaro R, Bahjat FR, Hotchkiss R, Clare-Salzler M, Moldawer LL (2004) Characterization of the systemic loss of dendritic cells in murine lymph nodes during polymicrobial sepsis. J Immunol 173:3035–3043
Tinsley KW, Grayson MH, Swanson PE, Drewry AM, Chang KC, Karl IE, Hotchkiss RS (2003) Sepsis induces apoptosis and profound depletion of splenic interdigitating and follicular dendritic cells. J Immunol 171:909–914
Hotchkiss RS, Tinsley KW, Swanson PE, Grayson MH, Osborne DF, Wagner TH, Cobb JP, Coopersmith C, Karl IE (2002) Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol 168:2493–2500
Alvarez D, Vollmann EH, von Andrian UH (2008) Mechanisms and consequences of dendritic cell migration. Immunity 29:325–342
Grabbe S, Kampgen E, Schuler G (2000) Dendritic cells: multi-lineal and multi-functional. Immunol Today 21:431–433
Gilliet M, Cao W, Liu YJ (2008) Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol 8:594–606
Guisset O, Dilhuydy MS, Thiebaut R, Lefevre J, Camou F, Sarrat A, Gabinski C, Moreau JF, Blanco P (2007) Decrease in circulating dendritic cells predicts fatal outcome in septic shock. Intensive Care Med 33:148–152
Bone RC, Sprung CL, Sibbald WJ (1992) Definitions for sepsis and organ failure. Crit Care Med 20:724–726
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent J-L, Ramsay G, SCCM/ESICM/ACCP/ATS/SIS (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med 31:1250–1256
Caille V, Chiche JD, Nciri N, Berton C, Gibot S, Boval B, Payen D, Mira JP, Mebazaa A (2004) Histocompatibility leukocyte antigen-D related expression is specifically altered and predicts mortality in septic shock but not in other causes of shock. Shock 22:521–526
Mermel LA, Farr BM, Sherertz RJ, Raad II, O’Grady N, Harris JS, Craven DE (2001) Guidelines for the management of intravascular catheter-related infections. Clin Infect Dis 32:1249–1272
American Thoracic Society; Infectious Diseases Society of America (2005) Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171:388–416
Vuckovic S, Gardiner D, Field K, Chapman GV, Khalil D, Gill D, Marlton P, Taylor K, Wright S, Pinzon-Charry A, Pyke CM, Rodwell R, Hockey RL, Gleeson M, Tepes S, True D, Cotterill A, Hart DN (2004) Monitoring dendritic cells in clinical practice using a new whole blood single-platform TruCOUNT assay. J Immunol Methods 284:73–87
Hosmalin A, Lichtner M, Louis S (2008) Clinical analysis of dendritic cell subsets: the dendritogram. Methods Mol Biol 415:273–290
Poehlmann H, Schefold JC, Zuckermann-Becker H, Volk HD, Meisel C (2009) Phenotype changes and impaired function of dendritic cell subsets in patients with sepsis: a prospective observational analysis. Crit Care 13:R119
Monneret G, Lepape A, Voirin N, Bohe J, Venet F, Debard AL, Thizy H, Bienvenu J, Gueyffier F, Vanhems P (2006) Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med 32:1175–1183
Castera L, Hatzfeld-Charbonnier AS, Ballot C, Charbonnel F, Dhuiege E, Velu T, Formstecher P, Mortier L, Marchetti P (2009) Apoptosis-related mitochondrial dysfunction defines human monocyte-derived dendritic cells with impaired immuno-stimulatory capacities. J Cell Mol Med 13:1321–1335
Pugin J (2008) Dear SIRS, the concept of “alarmins” makes a lot of sense! Intensive Care Med 34:218–221
D’Arpa N, Accardo-Palumbo A, Amato G, D’Amelio L, Pileri D, Cataldo V, Mogavero R, Lombardo C, Napoli B, Conte F (2009) Circulating dendritic cells following burn. Burns 35:513–518
Cavaillon JM, Annane D (2006) Compartmentalization of the inflammatory response in sepsis and SIRS. J Endotoxin Res 12:151–170
Rossi M, Young JW (2005) Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J Immunol 175:1373–1381
Lyakh L, Trinchieri G, Provezza L, Carra G, Gerosa F (2008) Regulation of interleukin-12/interleukin-23 production and the T-helper 17 response in humans. Immunol Rev 226:112–131
Benjamim CF, Lundy SK, Lukacs NW, Hogaboam CM, Kunkel SL (2005) Reversal of long-term sepsis-induced immunosuppression by dendritic cells. Blood 105:3588–3595
Pastille E, Didovic S, Brauckmann D, Rani M, Agrawal H, Schade FU, Zhang Y, Flohe SB (2011) Modulation of dendritic cell differentiation in the bone marrow mediates sustained immunosuppression after polymicrobial sepsis. J Immunol 186:977–986
Pene F, Zuber B, Courtine E, Rousseau C, Ouaaz F, Toubiana J, Tazi A, Mira JP, Chiche JD (2008) Dendritic cells modulate lung response to Pseudomonas aeruginosa in a murine model of sepsis-induced immune dysfunction. J Immunol 181:8513–8520
Benjamim CF, Hogaboam CM, Lukacs NW, Kunkel SL (2003) Septic mice are susceptible to pulmonary aspergillosis. Am J Pathol 163:2605–2617
Landelle C, Lepape A, Voirin N, Tognet E, Venet F, Bohe J, Vanhems P, Monneret G (2010) Low monocyte human leukocyte antigen-DR is independently associated with nosocomial infections after septic shock. Intensive Care Med 36:1859–1866
Pene F, Courtine E, Ouaaz F, Zuber B, Sauneuf B, Sirgo G, Rousseau C, Toubiana J, Balloy V, Chignard M, Mira JP, Chiche JD (2009) Toll-like receptors 2 and 4 contribute to sepsis-induced depletion of spleen dendritic cells. Infect Immun 77:5651–5658
Claessens YE, Fontenay M, Pene F, Chiche JD, Guesnu M, Hababou C, Casadevall N, Dhainaut JF, Mira JP, Cariou A (2006) Erythropoiesis abnormalities contribute to early onset anemia in patients with septic shock. Am J Respir Crit Care Med 174:51–57
Acknowledgments
This work was supported by grants from the European Society of Intensive Care Medicine and the Société de Réanimation de Langue Française. We thank Prof. A. Brezin from the Department of Ophthalmology of Cochin Hospital for the opportunity given to us to enroll the control patients. We are grateful to Dr. C. Elie for her insightful appraisal of the statistical methods used in this study.
Conflict of interest
None of the authors have any financial interests with commercial organizations whose products are related to the subject matter of this manuscript (no existing conflicts of interest).
Author information
Authors and Affiliations
Corresponding author
Additional information
D. Grimaldi and S. Louis contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Grimaldi, D., Louis, S., Pène, F. et al. Profound and persistent decrease of circulating dendritic cells is associated with ICU-acquired infection in patients with septic shock. Intensive Care Med 37, 1438–1446 (2011). https://doi.org/10.1007/s00134-011-2306-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2306-1