Abstract
Purpose
To determine the feasibility of daily titration of the neurally adjusted ventilatory assist (NAVA) level in relation to the maximal diaphragmatic electrical activity (EAdimaxSBT) measured during a spontaneous breathing trial (SBT) during pressure support ventilation (PSV).
Methods
The study included 15 consecutive patients in whom mechanical ventilation weaning was initiated with the NAVA mode. EAdimaxSBT was determined daily during an SBT using PSV with 7 cmH2O of inspiratory pressure and no positive end-expiratory pressure (PEEP). If the SBT was unsuccessful, NAVA was used and the level was then adjusted to obtain an EAdi of ~60% of the EAdimaxSBT. Arterial blood gas analyses were performed 20 min after each change in NAVA level.
Results
Three patients were dropped from the study at day 4 because of worsening of their sickness. The median duration of NAVA ventilation was 4.5 days (IQR 3–6.5). From day 1 to extubation, EAdimaxSBT and EAdi increased significantly from 16.6 (9.6) to 21.7 (10.3) μV (P = 0.013) and from 10.0 (5.5) to 15.1 (9.2) μV (P = 0.026), respectively. The pressure delivered significantly decreased from 20 (8) to 10 (5) cmH2O (P = 0.003). Conversely, tidal volume, carbon dioxide tension, and pH values remained unchanged during the same period.
Conclusion
These results suggest that daily titration of NAVA level with an electrical goal of ~60% EAdimaxSBT is feasible and well tolerated. The respiratory mechanics improvement and increase in respiratory drive allowed for a daily reduction of the NAVA level while preserving breathing, oxygenation, and alveolar ventilation until extubation.
Similar content being viewed by others
Introduction
The start of the weaning process from mechanical ventilation requires the resumption of neuromuscular activity to stimulate the respiratory system to meet metabolic demands and maintain carbon dioxide homeostasis. Currently, the established mode used in the weaning of patients from mechanical ventilation is pressure support ventilation (PSV) [1]. During this ventilatory mode, the level of pressure is fixed and adapted to achieve a tidal volume (V T) between 6 and 8 ml/kg [2]. The process of weaning a patient from mechanical ventilation using PSV usually includes a daily spontaneous breathing trial (SBT), which reduces the duration of mechanical ventilation in diverse populations of patients after acute respiratory failure [2–4].
Neurally adjusted ventilatory assist (NAVA) is ventilatory mode which provides pressure in proportion to the electrical activity of the diaphragm. Ventilator support is initiated with the detection of the diaphragmatic neural drive while the pressure assistance is automatically delivered in proportion to the EAdi intensity [5, 6]. Support is then cycled off with the termination of the respiratory output by the respiratory centers [7, 8]. With NAVA, the amount of pressure applied by the ventilator to the airway opening throughout inspiration is determined by the processed EAdi, expressed in μV, multiplied by a user-controlled gain factor (“NAVA level”), whose unit is cmH2O/μV. Recent studies have demonstrated that NAVA has beneficial effects compared with standard PSV, as it can improve patient–ventilator synchrony in intubated spontaneously breathing intensive care patients [9] and oxygenation in postoperative patients [10]; but no one knows how to adjust the NAVA level.
With PSV the patient may produce a small and brief effort to breathe, just sufficient to trigger the ventilator and then relax, causing the patient to be passively ventilated for a large majority of the inspiratory phase and not in control of their breathing pattern. With NAVA, increasing the NAVA level from zero to a high level in healthy subjects can also partially unload respiratory muscle [7]. In a recent clinical study, Coisel et al. [10] proposed the initial NAVA level setting based on the inspiratory pressure level which was required to obtain a volumetric goal of V T between 6 and 8 ml/kg of ideal body weight; however, this approach can be difficult as they described an important variability of V T under NAVA in comparison to PSV. Another way to adapt the NAVA level for each patient has been studied. Brander et al. [11] tested a titration of the NAVA level based on both esophageal pressure time product (PTP) and EAdi. It has been also proposed to use EAdi for adapting PSV with closed-loop control of respiratory drive [12]. These findings suggest that the NAVA level could be adjusted to target an EAdi relative to the highest value obtained during a standardized intervention. Daily SBT with PSV 7/0 [pressure support of 7 cmH2O and no positive end-expiratory pressure (PEEP)] is a reproducible effort where EAdi can easily be measured. A mean respiratory effort ~60% of the value developed during the inspiratory effort could be adapted to prevent respiratory muscle fatigue or limit the incidence of over-assistance [13, 14]. Patients in whom PSV 7/0 can be maintained for two consecutive hours are usually extubated [1]. In patients who did not succeed in SBT, we tested the hypothesis that the NAVA level could be adapted for each patient according to their individual highest EAdi during the daily SBT (EAdimaxSBT). We proposed a protocol in which the NAVA level was titrated daily to obtain an EAdi of ~60% of the EAdimaxSBT value. The primary goal of this study was to evaluate the feasibility of the proposed protocol as evidenced by stable respiratory parameters, arterial blood gases, and cardiopulmonary function 20 min after each modification of the NAVA level.
Methods
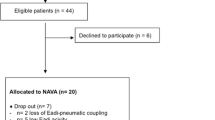
This observational study was approved by the institutional review board (Comité de Protection des Personnes Sud-Ouest et Outre Mer III) of the Centre Hospitalier Universitaire (CHU) Bordeaux and was conducted in the Department of Anesthesia and Critical Care between November 2009 and June 2010. Informed consent was obtained. Patients mechanically ventilated for more than 4 days who were going to be weaned from mechanical ventilation were included in the study. Patients were ventilated with a SERVO-i ventilator in the NAVA mode (maquet Criticial Care, Solna, Sweden). A heated humidifier was used for gas conditioning. As previously described, EAdi was obtained through a nasogastric tube with a multiple array of electrodes placed at its distal end (EAdi catheter, Maquet Critical Care, Solna, Sweden) [6, 15]. Correct positioning of the EAdi catheter was ensured by means of a specific function of the ventilator (“EAdi catheter positioning”) [16]. Patients were initially switched from volume cycled ventilation (VCV) to PSV for 1 h (Fig. 1). The fixed level of pressure support was adapted to obtain a V T of 7 ml/kg of ideal body weight. The EAdi was recorded. After 1 h, an SBT was performed by using PSV with 7 cmH2O of pressure without PEEP. SBT failure was defined as the development of hypoxemia (SpO2 < 88% for ≥5 min), abrupt changes in mental status, an acute cardiac arrhythmia, or two or more signs of respiratory distress, including tachycardia (>130 bpm), bradycardia (<50 bpm), use of accessory muscles, abdominal paradox, diaphoresis, or marked dyspnea. Patients who failed the SBT were reventilated immediately using the settings employed prior to the trial. If SBT was well tolerated PSV was continued for at least 2 h and extubation was discussed, otherwise the NAVA mode was used. In this case, the NAVA level was titrated to obtain EAdi values of ~60% of the highest EAdi measured during SBT (EAdimaxSBT). As shown in Fig. 2, EAdimaxSBT was measured during an SBT. If the EAdi was inferior to 60% of the EAdimaxSBT, the NAVA level was decreased. If EAdi was superior to 60% of the EAdimaxSBT, the NAVA level was increased. NAVA level modification was done only once a day after the SBT. An analysis of arterial blood gases was performed 20 min after each modification of the NAVA level, but also later in case a modification of respiratory setting was necessary. If the modification of NAVA level was not well tolerated because of hypoxemia or a decrease of pH with an increase of PaCO2 the previous NAVA level was restored. All ventilatory and hemodynamic data were recorded during the analysis of arterial blood gases. The highest EAdimaxSBT noticed was confirmed on the trends of the SERVO-i ventilator (Fig. 3).
Trends in two different patients as illustrated on the SERVO-i screen over 1 h. Each column represents the mean value over 1 min. For the first patient we can see EAdi variations before, during, and after an SBT. EAdimaxSBT was 38 μV after an SBT of 8 min and allowed reduction of the NAVA level from 1.5 to 1 cmH2O/μvolts in order to have EAdi values after the SBT around 23 μV (60% of EAdimaxSBT). Arterial blood gases, before and 15 min after NAVA level modification, were unchanged (PaO2 92 vs. 90 mmHg and PaCO2 41.2 vs. 39 mmHg, respectively). The second patient did not tolerate the SBT and EAdimaxSBT increased immediately to 15 μV
Statistical analysis
Data are expressed as mean (SD) and median [interquartile range (IQR)] for non-Gaussian variables (e.g., duration of controlled ventilation and of ventilatory weaning by NAVA). The normal distribution of continuous variable was assessed by using skewness and kurtosis statistical tests. Comparison of several means used one-way analysis of variance for repeated measurements. Post hoc test analysis was performed using the Newman–Keuls test. The comparison of two means was performed by using the paired Student t test. All P values were two-tailed and a P value less than 0.05 was required to reject the null hypothesis. Statistical analysis was performed with NCSS (Statistical Solutions Ltd., Cork, Ireland).
Results
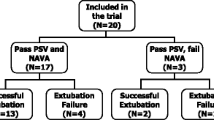
Fifteen patients were enrolled in the study. Baseline characteristics are summarized in Table 1. Three patients were dropped from the study at day 3 because of worsening of their sickness. After the initial ventilation using PSV for 1 h, EAdi values were 35 (15)% of EAdimaxSTB. The NAVA level at initiation was 2.6 (1.6). The switch from PSV to the NAVA mode at day 1 was associated with an increase in EAdi and ventilator respiratory frequency (RF) (Table 2). Conversely, systolic arterial pressure remained unchanged. During the first 3 days of NAVA ventilation, the NAVA level could be significantly decreased (Table 3). Between day 1 of the NAVA mode and just prior to extubation (Table 4), EAdimax and EAdi increased significantly from 16.6 (9.6) to 21.7 (10.3) μV (P = 0.012) and from 10.0 (5.5) to 15.1 (9.2) μV (P = 0.026), respectively. The level of inspiratory pressure over PEEP significantly decreased from 20 (8) to 10 (5) cmH2O (P = 0.003). Conversely, tidal volume, carbon dioxide tension, and pH values remained unchanged during the same period. No patients succeeded in an SBT of 2 h at days 1 and 2, but 5 patients succeeded at day 3 and were successfully extubated. Three patients were still intubated at 1 week. The median duration of volume-controlled ventilation before NAVA and of weaning by NAVA ventilatory mode was 10 days (IQR 9–27) and 4.5 (IQR 3–6.5), respectively. One patient was re-intubated within 4 days following extubation because of pulmonary edema.
Discussion
The major finding of this study is the feasibility of the daily evaluation of EAdimax with an SBT and the adjustment of the NAVA level. Each modification of the NAVA level, according to the proposed protocol, satisfied the respiratory demand of patients as evidenced by stable respiratory parameters, arterial blood gases, and cardiopulmonary function.
The present work proposed a way for setting the NAVA level according to an electrical goal (EAdimaxSBT) rather than to a volumetric goal (expired V T). This protocol demonstrates that the most clinically relevant interpretation of EAdi is its daily variation during an objective and reproducible inspiratory effort rather than by its value alone. The daily SBT with PSV 7 and no PEEP was chosen for this study as a reproducible inspiratory effort, but other techniques could be substituted provided that they were routinely performed and reproducible. Indeed in this study, EAdimaxSBT does not represent the highest EAdi value as EAdi without any assist at all or with a clamp on the tracheal tube would be higher, but we never use these tests.
The NAVA level observed in our study when the NAVA mode was initiated seems closely similar to those recently reported by Coisel et al. [10], but differs from those found by other investigators [11]. These differences could likely be explained by differences in study population characteristics and the method of setting the NAVA level [10, 11] The high level of pressure support that we could observe in our study might be related to the severity of our patients’ conditions. The mean duration of volume-controlled ventilation that preceded the ventilatory weaning was extensive and pulmonary mechanics were compromised as 4 patients had extremely severe H1N1 ARDS and exhibited a thoracopulmonary compliance less than 10 ml/cmH2O and required extracorporeal membrane oxygenation (ECMO). Two other patients with ARDS had pancreatitis with abdominal compartment syndrome and one patient had exacerbation of cystic fibrosis and required mechanical ventilation prior to lung transplantation. Three patients could be defined as difficult to wean as they were unable to sustain a prolonged SBT at day 3 and were still ventilated after 7 days [1].
This protocol used a titration of the NAVA level in order to have an EAdi around 60% of EAdimaxSBT. This level was arbitrarily chosen on the basis of protocols of muscular reeducation using electromyogram [10] and according to studies evaluating diaphragm activation during exercise [13] and NAVA level titration with EAdi and PTP measurements [11, 17]. Brander et al. [11] suggested that the optimal NAVA level (or adequate NAVA level) was at the inflection point of the airway pressure trend during a stepwise increase in the NAVA level. Interestingly they found it to be around 75% of the highest EAdi obtained at NAVA level zero (PEEP was present) [11]. We believe that with an adequate NAVA level, the upper limit of EAdi should be around 60% of the maximum EAdi during the SBT which is close to the resting diaphragm activation observed in stable COPD patients after incremental exhaustive bicycle exercise [13]. The findings suggest that the NAVA level that we chosen is likely to be sufficient to prevent muscle fatigue. Using EAdi in order to adapt PSV with closed-loop control of respiratory drive has been already proposed by Spahija et al. [12] They established a target range of EAdi, and let breath by breath changes in the EAdi above or below a certain range determine an increase or reduction of PSV.
An interesting result in our study was the daily increase in EAdimaxSBT (Fig. 4). This finding was unexpected and raises many questions for which answers can be only speculative. The day 1 to day 3 increase of EAdimaxSBT was associated with an improvement of the respiratory mechanic of studied patients, as half of them were about to be successfully extubated at day 3. Moreover, while EAdimaxSBT was higher at day 3, V T (IBW) stayed stable with a lower level of inspiratory pressure over PEEP. This improvement allowed us to reduce the NAVA level while preserving both breathing and gas exchange. A residual sedative effect could participate in a decreased EAdimaxSBT when ventilatory weaning was initiated. Indeed, sedative drugs such as midazolam decrease respiratory drive but also diaphragmatic contraction. Consequently, elimination of sedative drugs between day 1 and 3 may be associated with an improvement of respiratory drive and thus EAdimaxSBT values [18]. Flumazenil could have been tested at day 1 in order to quantify EAdi modification [19]. It is also a common observation that surface electromyographic (EMG) amplitude, thus net neural drive to a muscle, increase within days of training using isometric, concentric, or other forms of contraction (more motor units could be recruited or firing faster) [20]. With an interval of 24 h between each EAdimaxSBT measurement, diaphragmatic function may have changed while recovering from mechanical ventilation injury [21–23]. It is possible that the reserve of the diaphragm increases, and that after 3 days diaphragm contributes more to ventilation than the accessory muscles. Of course, as no any additional measurements have been performed, these explanations are purely speculative. If the reason is only a decrease of residual sedative drugs at the beginning of the weaning process, then EAdi monitoring and daily measurement of EAdi with the same level of pressure are useful as they unmask this side effect and allow the NAVA level to be adapted. Another beneficial aspect of this protocol was that patients were able to increase their EAdi and proportional assistance by at least 40% in the event of an increase in breathing effort because of secretions in the tube, mobilization, or other clinical situations.
The value of EAdi varied greatly between patients, confirming the hypothesis that the value of EAdi itself is not correlated with diaphragm strength, but more with its variation over time. Some patients at day 1 had higher EAdi values than other patients on the day of extubation. Patients after lung transplantation were not different from the others [15].
At day 1, PSV was used as a bridge between volume-controlled ventilation and NAVA in order to measure EAdi with a V T of 7 ml/kg of ideal body weight. We could observe that EAdi values were dramatically low after 1 h of PSV, about one-third of the EAdimax measured during the SBT. This raised a question about whether or not these patients were over-assisted.
Once the NAVA level was stabilized, the majority of patients, having a high level of pressure delivery, benefited from the better synchronization of NAVA [10]. NAVA improves patient–ventilator synchrony by reducing the triggering and cycling delays, especially at higher levels of assist [9, 24]. The fact that pressure is delivered in proportion to the electrical activity of the diaphragm explains the variability observed between the two SBT pressure assist methods [10, 25]. A beneficial aspect of this protocol in terms of the duration of mechanical ventilation was that an SBT was performed every day, beginning on day 1, to measure EAdimax [3, 4]. Some patients had a successful SBT 2 h in duration, whereas their level of pressure assist prior to the SBT was still relatively high (12 cmH2O). With NAVA, a daily SBT was sufficient to determine when extubation was feasible. Only one patient was reintubated within the 4 days following extubation because of pulmonary edema.
The following points should be considered in assessing the clinical relevance of this study. This was a feasibility study in which only 15 patients were enrolled. However, to our knowledge, it is the first study that evaluates the titration of NAVA guided by a standardized intervention effort during the entire weaning process. Further clinical trials are required to clarify whether or not NAVA employing this type of protocol represents an advantage compared with other forms of partial support in general and PSV in particular. This study only examined the feasibility of this specific protocol in order to determine an adapted NAVA level, which is difficult to find, for each patient everyday. Indeed, although NAVA is a new mode, the setting performed by the physician is “fixed” (NAVA level) and needs to be adapted everyday as in PSV.
Conclusion
A simple protocol was proposed to titrate the NAVA level daily with an electrical goal (EAdimaxSBT) rather than a V T. The EAdimaxSBT is measured during a fixed SBT which may be considered as an objective and standardized intervention. The improvement of respiratory mechanics and diaphragm activity allowed a daily reduction of the NAVA level while preserving breathing and maintaining blood gases until a successful SBT was conducted. Further studies are still warranted to clearly demonstrate the clinical benefits of such a protocol and compare it with other ventilatory weaning modes such as PSV.
References
Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, Pearl R, Silverman H, Stanchina M, Vieillard-Baron A, Welte T (2007) Weaning from mechanical ventilation. Eur Respir J 29:1033–1056
Esteban A, Ferguson ND, Meade MO, Frutos-Vivar F, Apezteguia C, Brochard L, Raymondos K, Nin N, Hurtado J, Tomicic V, González M, Elizalde J, Nightingale P, Abroug F, Pelosi P, Arabi Y, Moreno R, Jibaja M, D’Empaire G, Sandi F, Matamis D, Montañez AM, Anzueto A, VENTILA Group (2008) Evolution of mechanical ventilation in response to clinical research. Am J Respir Crit Care Med 177:170–177
Kollef MH, Shapiro SD, Silver P, St John RE, Prentice D, Sauer S, Ahrens TS, Shannon W, Baker-Clinkscale D (1997) A randomized, controlled trial of protocol-directed versus physician-directed weaning from mechanical ventilation. Crit Care Med 25:567–574
Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, Taichman DB, Dunn JG, Pohlman AS, Kinniry PA, Jackson JC, Canonico AE, Light RW, Shintani AK, Thompson JL, Gordon SM, Hall JB, Dittus RS, Bernard GR, Ely EW (2008) Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomized controlled trial. Lancet 371:126–134
Sinderby CA, Beck JC, Lindström LH, Grassino AE (1997) Enhancement of signal quality in esophageal recordings of diaphragm EMG. J Appl Physiol 82:1370–1377
Sinderby C, Navalesi P, Beck J, Skrobik Y, Comtois N, Friberg S, Gottfried SB, Lindström L (1999) Neural control of mechanical ventilation in respiratory failure. Nat Med 5:1433–1436
Sinderby C, Beck J, Spahija J, de Marchie M, Lacroix J, Navalesi P, Slutsky AS (2007) Inspiratory muscle unloading by neurally adjusted ventilatory assist during maximal inspiratory efforts in healthy subjects. Chest 131:711–717
Colombo D, Cammarota G, Bergamaschi V, De Lucia M, Corte FD, Navalesi P (2008) Physiologic response to varying levels of pressure support and neurally adjusted ventilatory assist in patients with acute respiratory failure. Intensive Care Med 34:2010–2018
Piquilloud L, Vignaux L, Bialais E, Roeseler J, Sottiaux T, Laterre PF, Jolliet P, Tassaux D (2010) Neurally adjusted ventilatory assist improves patient–ventilator interaction. Intensive Care Med 37:263–271
Coisel Y, Chanques G, Jung B, Constantin JM, Capdevila X, Matecki S, Grasso S, Jaber S (2010) Neurally adjusted ventilatory assist in critically ill postoperative patients: a crossover randomized study. Anesthesiology 113:925–935
Brander L, Leong-Poi H, Beck J, Brunet F, Hutchison SJ, Slutsky AS, Sinderby C (2009) Titration and implementation of neurally adjusted ventilatory assist in critically ill patients. Chest 135:695–703
Spahija J, Beck J, de Marchie M, Comtois A, Sinderby C (2005) Closed-loop control of respiratory drive using pressure-support ventilation: target drive ventilation. Am J Respir Crit Care Med 171:1009–1014
Sinderby C, Spahija J, Beck J, Kaminski D, Yan S, Comtois N, Sliwinski P (2001) Diaphragm activation during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 163:1637–1641
Portero P, Bigard AX, Gamet D, Flageat JR, Guézennec CY (2001) Effects of resistance training in humans on neck muscle performance, and electromyogram power spectrum changes. Eur J Appl Physiol 84:540–546
Rozé H, Janvier G, Ouattara A (2010) Cystic fibrosis patient awaiting lung transplantation ventilated with neurally adjusted ventilatory assist. Br J Anaesth 105:97–98
Barwing J, Ambold M, Linden N, Quintel M, Moerer O (2009) Evaluation of the catheter positioning for neurally adjusted ventilatory assist. Intensive Care Med 35:1809–1814
Carteau G, Lyazidi A, Thille AW, Brochard L (2010) Comparison of a pressure support titrations with NAVA and PSV during the weaning process from mechanical ventilation. French Congress of Intensive Care. SRLF 2010. abstract SP 300
Gross JB, Zebrowski ME, Carel WD, Gardner S, Smith TC (1983) Time course of ventilatory depression after thiopental and midazolam in normal subjects and in patients with chronic obstructive pulmonary disease. Anesthesiology 58:540–544
Gross JB, Blouin RT, Zandsberg S, Conard PF, Häussler J (1996) Effect of flumazenil on ventilatory drive during sedation with midazolam and alfentanil. Anesthesiology 85:713–720
Sassoon CS, Caiozzo VJ, Manka A, Sieck GC (2002) Altered diaphragm contractile properties with controlled mechanical ventilation. J Appl Physiol 92:2585–2595
Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrager JB (2008) Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 358:1327–1335
Petrof BJ, Jaber S, Matecki S (2010) Ventilator-induced diaphragmatic dysfunction. Curr Opin Crit Care 16:19–25
Felici F (2006) Neuromuscular responses to exercise investigated through surface EMG. J Electromyogr Kinesiol 16:578–585
Spahija J, de Marchie M, Albert M, Bellemare P, Delisle S, Beck J, Sinderby C (2010) Patient–ventilator interaction during pressure support ventilation and neurally adjusted ventilatory assist. Crit Care Med 38:518–526
Schmidt M, Demoule A, Cracco C, Gharbi A, Fiamma MN, Straus C, Duguet A, Gottfried SB, Similowski T (2010) Neurally adjusted ventilatory assist increases respiratory variability and complexity in acute respiratory failure. Anesthesiology 112:670–681
Acknowledgments
The authors would like to thank Erwan Floch, PharmD (Newmed Publishing Services) for reviewing the manuscript, Julie Boussuge PharmD (DRCI), Olivier Branchard and all the physiotherapists for their assistance. Each author is a member of the medical staff of the department and has played a key role in the study. We also thank the nursing staff of the thoracic intensive care unit for their valuable cooperation. This study was supported solely by the Department of Anesthesia and Critical Care.
Conflict of interest
No conflict of interest has been declared by the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was presented in part at the 2010 Annual Meeting of the French Society of Anesthesiology and Critical Care, Paris, September 23, 2010.
Rights and permissions
About this article
Cite this article
Rozé, H., Lafrikh, A., Perrier, V. et al. Daily titration of neurally adjusted ventilatory assist using the diaphragm electrical activity. Intensive Care Med 37, 1087–1094 (2011). https://doi.org/10.1007/s00134-011-2209-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2209-1