Abstract
Objective
We aim to evaluate the incidence and outcome of acute kidney injury (AKI) among critically ill adult patients with H1N1 2009 infection.
Design and patients
From a prospectively collected influenza A (H1N1) 2009 bi-national, we identified 671 adult patients admitted to intensive care unit (ICU) from June 1 to August 31, 2009. Of these, 628 (93.6%) had admission and/or peak serum creatinine values during ICU stay. We defined AKI according to the creatinine criteria of the RIFLE classification.
Results
Of 628 adult patients, 211 [33.6%, 95% confidence interval (CI) 29.8–37.4%] had AKI: 41 (6.5%) risk, 56 (8.9%) injury and 114 (18.2%) failure. Of all 211 AKI patients, 76 [36.0% (29.4–42.6%)] died in hospital (36.6% in risk, 25.0% in injury and 41.3% in failure group) compared with 33 of 408 (8.1%) patients without AKI. Among the 33 AKI patients treated with renal replacement therapy, 13 died (39.4%). Mechanical ventilation [odds ratio (OR) 3.62 (2.07–6.34)], any severe co-morbidity (OR 2.36, 95% CI 1.15–3.71), age (OR 1.02, 95% CI 1.01–1.03 per 1 year increase), and AKI (OR 6.69, 95% CI 4.25–10.55) were independently associated with hospital mortality.
Conclusions
Acute kidney injury appears common in H1N1 2009 infected patients and is independently associated with an increased risk of hospital mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An influenza A (H1N1) 2009 pandemic emerged during 2009 and caused a significant burden of critical illness [1–3]. Little information is available on how such illness affected the kidney. Influenza A infections rarely cause acute kidney injury (AKI), mostly due to rhabdomyolysis [4, 5]. AKI has been reported in 17% of patients with avian influenza A (H5N1) [6], and, recently, a small single-centre case series of H1N1 2009 patients suggested a high risk (32%) of AKI [7]. More recently, a multicentre observational study of 50 critically ill patients with H1N1 infection reported a 67% incidence of AKI [8]. However, so far, the evidence is limited and no large, prospective, multicentre studies of H1N1 have reported on the incidence and outcome of AKI.
Accordingly, we used a prospectively collected influenza A (H1N1) 2009 bi-national [2] to evaluate the incidence and hospital outcome of AKI among critically ill adult patients with H1N1 2009 infection.
Materials and methods
Patients
We have previously performed a multicentre inception cohort study in 187 ICUs in Australia and New Zealand comprising all adult, paediatric and combined adult and paediatric ICUs in Australia and New Zealand [2]. Each centre obtained Institutional Ethics Committee approval, and requirement for individual subject informed consent was waived at all sites. Between June 1 and August 31, 2009, all patients admitted to ICU with confirmed influenza A were identified. Influenza A was confirmed by polymerase chain reaction (PCR), antigen detection or serology. From this prospectively collected database, we then identified all adult patients admitted with H1N1 2009. Of these patients, we selected those who had admission and/or peak creatinine values. We defined AKI according to the creatinine criteria of the RIFLE classification [9]. We report our findings according to the STROBE guidelines for observational studies [10].
Data collection
We collected the following patient-specific data: hospital and ICU admission date and time, age, gender, for women whether pregnant or postpartum <28 days, co-morbidities [any Acute Physiology and Chronic Health Evaluation (APACHE) III co-morbidity], history of chronic diseases, and airway status at ICU admission [2]. In addition, we collected data on daily use of renal replacement therapy (RRT) and mechanical ventilation, and serum creatinine and serum creatine kinase [normal values <200 IU/L] values at ICU admission and peak value during ICU stay. We recorded patient outcomes at hospital discharge status or as still in hospital as of November 23, 2009.
Data management and statistical methods
We collected data using electronic case report forms. The study-coordinating centre was the Australian and New Zealand Intensive Care Research Centre, Monash University, Melbourne, Australia. When the same patient was transferred between ICUs they were counted as a single ICU admission. We made no assumptions for missing data, and calculated all proportions as percentages of available data.
We identified AKI using the RIFLE [9] classification during ICU stay as recommended [11, 12] by the Acute Dialysis Quality Initiative group [9], and validated [13]. We identified the incidence of admission AKI using the first admission creatinine value, and defined “later” AKI if AKI developed during ICU stay in those patients with normal creatinine values at ICU admission. We used a cut-off value of 5,000 IU/L for markedly elevated creatine kinase as suggested previously [14].
We performed statistical analysis using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA). We calculated descriptive statistics for all study variables. We report continuous variables as median with interquartile range (IQR) and categorical variables as percentage with 95% confidence interval (95% CI) where appropriate. We performed univariate analysis for hospital mortality using chi-square, Fisher’s exact test or Wilcoxon rank-sum test as appropriate. We performed multivariate logistic regression analysis to identify factors independently associated with an increased risk of hospital mortality, using a multivariate model constructed using both stepwise selection and backwards elimination techniques. We included all variables with p < 0.10 in the univariate model in the model selection process. Two-sided p value <0.05 was considered to be statistically significant, except for the multivariate model where p value <0.01 was used.
Results
Incidence and severity of AKI
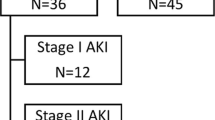
We identified 671 adult patients with H1N1 infection admitted to ICU from June 1 to August 31, 2009. Of these, 628 (93.6%) had admission (N = 589, 87.7%) and/or peak serum creatinine (N = 567, 84.5%) values measured during their ICU stay. Among these adult patients, we identified 211 (33.6%, 95% CI 29.8–37.4%) H1N1 patients with AKI according to the RIFLE classification: 41 (6.5%) risk, 56 (8.9%) injury and 114 (18.2%) failure (Fig. 1, Table 1).
Of 211 AKI patients, 95 (45.0%) without chronic renal failure (CRF) and 2 (1%) with CRF had AKI on ICU admission, while 104 (49.3%) without CRF and 10 (4.7%) with CRF developed AKI during their ICU stay. Of 97 patients with admission AKI, 53 of 94 (56.4%) received vasopressors and 74 of 96 (77.1%) received mechanical ventilation (data missing for 3 and 1, respectively). The corresponding numbers for 114 patients with later AKI were 61 of 101 (60.4%) (data missing for 13) and 97 of 114 (85.1%), respectively.
Creatine kinase
The median (IQR) creatine kinase (CK) was 677 IU/L (254–2,295) in AKI and 413 IU/L (139–1,170) in non-AKI patients (p = 0.006). Median CK was 528, 638 and 918 IU/L in RIFLE classes R, I and F, respectively (p = 0.04). Of 120 AKI patients with available data on CK, 19 (15.8%) (2 in R, 3 in I and 14 in F class) had elevated serum creatine kinase exceeding 5,000 IU/L compared with 15 of 175 (8.6%) in patients without AKI (p = 0.06).
In-hospital mortality
Of all 211 AKI patients, 76 [36.0% (29.4–42.6%)] died in hospital (36.6% in RIFLE class R, 25.0% in I and 41.3% in F) compared with 33 of 408 (8.1%) H1N1 patients without AKI (Table 2). Of the 97 patients with admission AKI, 32 (33.0%) died in the hospital compared with 44 of 114 (38.6%) with later AKI (difference 5.6%, 95% CI 7.3–18.5%, ns). Of 33 RRT patients (15.6% of 211 AKI patients, 4.9% of all H1N1 patients), 13 (39.4%) died and 7 (21.2%) remained RRT dependent at hospital discharge. On multivariate analysis, mechanical ventilation, any severe co-morbidity and AKI were independently associated with an increased risk of hospital death (Table 3), but vasopressor use was not.
Discussion
In this prospective observational multicentre study, we identified 211 (34%) adult H1N1 2009 patients who developed AKI. We found that AKI patients had an increased risk of hospital death (36% versus 8%, adjusted OR 6.69) compared with patients without AKI. In adult H1N1 2009 patients, mechanical ventilation, any severe co-morbidity and AKI itself were independently associated with hospital mortality. AKI was associated with higher CK levels.
Few studies have reported cases of AKI associated with subtypes of influenza A [4–6, 15, 16]. Although the mechanism of AKI in influenza A infection remains unclear, evidence from case series has related it to rhabdomyolysis [14, 17]. Recent reports have also demonstrated elevated creatine kinase values in 62% [18] and approximately 75% [19] of critically ill H1N1 2009 patients. We found that creatine kinase exceeded 5,000 IU/L [14] in 16% of AKI patients, supporting the notion that muscle cell injury may contribute to the development of AKI in some H1N1 patients. Diabetes was also more common in AKI patients, as were use of vasopressors and mechanical ventilation, confirming that diabetes is a risk factor for AKI even in these patients and suggesting that, in some patients, AKI was part of multiple organ dysfunction.
An approximate assessment of the incidence of H1N1 2009-associated AKI has so far only been reported in 32% (9 of 28) of patients from a small case series in Argentina [7], and, more recently in 64% (32 of 50) of mechanically ventilated patients in Canada [8]. Furthermore, data from another larger case series, while not providing specific information, suggested a close to 10% incidence of AKI [19]. Given the sample size, our study provides the first robust estimate of the incidence of AKI (95% CI 30–37%) in adult critically ill patients with confirmed H1N1 2009 infection. This incidence is comparable to the 36% incidence of AKI reported in general ICU patients in the SOAP study [20], but lower than the 67% incidence of AKI in the cohort study by Hoste et al. [21], and lower than in critically ill patients with confirmed or probable H1N1 infection (67%, 95% CI 53–80%) using RIFLE criteria with urine output data [8].
Our findings suggest that the severity of AKI in H1N1 2009 patients is comparable to in other ICU patients [22, 23]. Hospital mortality rates were higher in the R-group but lower in I- and F-groups than reported in other critically ill patients [22]. However, the mortality risk associated with AKI in H1N1 2009 patients seems to be comparable to that seen in other critically ill patients [20] (reported unadjusted ORs 2.4, 4.1 and 6.4 [22] according to different RIFLE classes, respectively).
We found that, in H1N1 2009 patients, RIFLE stages R, I and F were independently associated with hospital mortality, similarly to in other critically ill patients [23]. In addition, we found that severe chronic illness and mechanical ventilation on ICU admission were independently related to hospital death, in agreement with a previous study of AKI in general ICU patients [23].
To our knowledge, this is the only large, bi-national, multicentre study focussing on AKI in critically ill patients with H1N1 2009 infection. On the other hand, it has some limitations. First, creatinine values at admission were available in only 88% of patients. However, we did not detect any major differences between those with creatinine values available and those without. For those patients where the baseline creatinine value was missing, we estimated it as previously recommended [12]. Second, we did not have urine output data, which would have enabled us to use the complete RIFLE criteria. Other studies suggest that the urine output criteria have limited impact [24]. However, one recent multicentre study showed significant influence of urinary output on incidence of AKI [25]. Some patients may be oliguric without a significant increase in serum creatinine. Thus, the detected incidence of AKI without urine output in this study may be an underestimate. Third, data required for acute physiologic or organ dysfunction scoring were not available. Fourth, as an observational study, our results can only show association and no causal relationship. Fifth, we may have missed some possible confounding factors, such as additional infections, type of fluid resuscitation or lead-time bias. Finally, we did not gather data on dose or timing on RRT. However, our focus was simply to establish the burden and outcome of AKI in these unique patients.
In conclusion, we have demonstrated that one-third of critically ill patients with confirmed H1N1 2009 had AKI and that 4.9% required RRT. In addition, we found that patients with AKI had higher CK levels and that AKI was independently associated with increased risk of hospital mortality.
References
Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators, Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, Forrest P, Gattas D, Granger E, Herkes R, Jackson A, McGuinness S, Nair P, Pellegrino V, Pettilä V, Plunkett B, Pye R, Torzillo P, Webb S, Wilson M, Ziegenfuss M (2009) Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA 302:1888–1895
ANZIC Influenza Investigators, Webb SA, Pettilä V, Seppelt I, Bellomo R, Bailey M, Cooper DJ, Cretikos M, Davies AR, Finfer S, Harrigan PW, Hart GK, Howe B, Iredell JR, McArthur C, Mitchell I, Morrison S, Nichol AD, Paterson DL, Peake S, Richards B, Stephens D, Turner A, Yung M (2009) Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med 361:1925–1931
Webb SA, Seppelt IM (2009) Pandemic (H1N1) 2009 influenza (“swine flu”) in Australian and New Zealand intensive care. Crit Care Resusc 11:170–172
Simon NM, Rovner RN, Berlin BS (1970) Acute myoglobinuria associated with type A2 (Hong Kong) influenza. JAMA 212:1704–1705
Shenouda A, Hatch FE (1976) Influenza A viral infection associated with acute renal failure. Am J Med 61:697–702
Yu H, Gao Z, Feng Z, Shu Y, Xiang N, Zhou L, Huai Y, Feng L, Peng Z, Li Z, Xu C, Li J, Hu C, Li Q, Xu X, Liu X, Liu Z, Xu L, Chen Y, Luo H, Wei L, Zhang X, Xin J, Guo J, Wang Q, Yuan Z, Zhou L, Zhang K, Zhang W, Yang J, Zhong X, Xia S, Li L, Cheng J, Ma E, He P, Lee SS, Wang Y, Uyeki TM, Yang W (2008) Clinical characteristics of 26 human cases of highly pathogenic avian influenza A (H5N1) virus infection in China. PLoS One 3:e2985
Raffo L (2009) Influenza A(H1N1) epidemic in Argentina. Experience in a National General Hospital (Hospital Nacional Alejandro Posadas). Medicina (B Aires) 69:393–423
Sood MM, Rigatto C, Zarychanski R, Komenda P, Sood AR, Bueti J, Reslerova M, Roberts D, Mojica J, Kumar A (2009) Acute kidney injury in critically ill patients infected with 2009 pandemic influenza A(H1N1): report from a Canadian province. Am J Kidney Dis 55:848–855
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, The ADQI workgroup (2004) Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204–R212
Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M, For the STOBE Initiative (2007) Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Epidemiology 18:805–835
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in Renal Disease Study Group. Ann Intern Med 130:461–470
National Kidney Foundation (2002) K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39:S1–S266
Bagshaw SM, Uchino S, Cruz D, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Oudemans-van Straaten HM, Ronco C, Kellum JA, Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators (2009) A comparison of observed versus estimated baseline creatinine for determination of RIFLE class in patients with acute kidney injury. Nephrol Dial Transpl 24:2739–2744
Bosch X, Poch E, Grau JM (2009) Rhabdomyolysis and acute kidney injury. N Engl J Med 361:62–72
Cunningham E, Kohli R, Venuto RC (1979) Influenza-associated myoglobinuric renal failure. JAMA 242:2428–2429
Morgensen JL (1974) Myoglobinuria and renal failure associated with influenza. Ann Intern Med 80:362–363
Ayala E, Kagawa FT, Wehner JH, Tam J, Upadhyay D (2009) Rhabdomyolysis associated with 2009 influenza A(H1N1). JAMA 302:1863–1864
Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quiñones-Falconi F, Bautista E, Ramirez-Venegas A, Rojas-Serrano J, Ormsby CE, Corrales A, Higuera A, Mondragon E, Cordova-Villalobos JA, INER Working Group on Influenza (2009) Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med 361:680–689
Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, Stelfox T, Bagshaw S, Choong K, Lamontagne F, Turgeon AF, Lapinsky S, Ahern SP, Smith O, Siddiqui F, Jouvet P, Khwaja K, McIntyre L, Menon K, Hutchison J, Hornstein D, Joffe A, Lauzier F, Singh J, Karachi T, Wiebe K, Olafson K, Ramsey C, Sharma S, Dodek P, Meade M, Hall R, Fowler RA, Canadian Critical Care Trials Group H1N1 Collaborative (2009) Critically Ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 302:1872–1879
Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL, Sepsis Occurrence in Acutely Ill Patients (SOAP) Investigators (2008) A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care 12:R74
Hoste EA, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, Kellum JA (2006) RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care 10:R73
Ricci Z, Cruz D, Ronco C (2008) The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int 73:538–546
Ostermann M, Chang RW (2007) Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med 35:1837–1843
Bagshaw SM, George C, Dinu I, Bellomo R (2008) A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transpl 23:1203–1210
Joannidis M, Metnitz B, Bauer P, Schusterschitz N, Moreno R, Druml W, Metnitz PG (2009) Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med 35:1692–1702
Conflict of interest
The authors declared no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the ANZIC Influenza Investigators* [see appendix (as Electronic Supplementary Material)] and Australian and New Zealand Intensive Care Society Clinical Trials Group.
This article is discussed in an editorial available at: doi:10.1007/s00134-011-2196-2; related material can be found at doi:10.1007/s00134-011-2167-7 and doi:10.1007/s00134-011-2183-7.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pettilä, V., Webb, S.A.R., Bailey, M. et al. Acute kidney injury in patients with influenza A (H1N1) 2009. Intensive Care Med 37, 763–767 (2011). https://doi.org/10.1007/s00134-011-2166-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2166-8