Abstract
Purpose
We investigated the effects of periodical high pressure breaths (SIGH) or biphasic positive pressure ventilation (BIPAP) during helmet continuous positive airway pressure (CPAP) in patients with acute hypoxic respiratory failure.
Methods
We used a recently developed electromechanical expiratory valve (TwinPAP®, StarMed, Mirandola, Italy), which is time-cycled between two customizable positive end-expiratory pressure (PEEP) levels. We studied 21 patients (67 ± 17 years old) undergoing helmet CPAP. Continuous flow CPAP system was set at 60 l/min flow rate while maintaining clinical FiO2 (51 ± 15%). Five steps, lasting 1 h each, were applied: (1) spontaneous breathing with PEEP 0 cmH2O (SB), (2) CPAP with PEEP 8 cmH2O (CPAPbasal), (3) low PEEP, 8 cmH2O, for 25 s and high PEEP, 25 cmH2O, for 5 s (SIGH), (4) low PEEP, 8 cmH2O, for 3 s and high PEEP, 20 cmH2O, for 3 s (BIPAP), (5) CPAP with PEEP 8 cmH2O (CPAPfinal). We randomized the sequence of SIGH and BIPAP.
Results
PaO2 was significantly higher during all steps compared to SB. When compared to CPAPbasal, both SIGH and BIPAP induced a further increase in PaO2. PaO2 during SIGH and BIPAP were not different. The oxygenation improvement was maintained during CPAPfinal.
Conclusions
Superimposed, nonsynchronized positive pressure breaths delivered during helmet CPAP by means of the TwinPAP® system may improve oxygenation in patients with acute hypoxemic respiratory failure, even at a rate as low as two breaths per minute.
Similar content being viewed by others
Introduction
Noninvasive ventilation (NIV) is increasingly used in the care of patients with acute respiratory failure [1]. One of the crucial issues affecting NIV outcome is the choice of interface. Compared to standard face mask, the helmet, a transparent plastic hood containing the patient’s entire head, shows important advantages such as better patient tolerance and less risk of skin lesions [2, 3], thus allowing longer and more continuous applications [4]. Given its technical characteristics, the helmet is particularly effective in delivering continuous positive airway pressure (CPAP) [4–8]. When applied by a continuous flow system, helmet CPAP is easy to assemble and allows application of CPAP even outside of the ICU [7].
In order to extend the advantages of helmets to patients who may benefit from some degree of ventilatory assistance that is not provided by CPAP alone, a few investigators have used the helmet to deliver pressure support ventilation (PSV) [2, 3, 9–13]. Unfortunately, when used to deliver PSV, the helmet exhibits some disadvantages as compared to facial masks [9, 11–13]. The high volume and compliance of the helmet dissipate part of the applied pressure causing a reduced efficacy in decreasing inspiratory muscle effort [9]. Inspiratory and expiratory triggers are delayed and a high degree of patient-ventilator asynchrony is common [9, 11–13]. Moreover, depending on the total amount of fresh gas flow through the helmet [14], the accumulation of CO2 inside the helmet is more problematic during PSV than during continuous flow CPAP systems [14, 15]. Achieving effective ventilator support with helmet PSV might therefore be particularly difficult [11, 16, 17], thus limiting the application of PSV by helmet to a few experienced centers [2, 3, 9, 11].
A possible way to extend the indications of the helmet while maintaining the advantages of continuous gas flow without needing optimal synchronization is suggested by the early development of airway pressure release ventilation (APRV) [18, 19] and biphasic positive airway pressure (BIPAP) [20, 21]. Several experimental [22] and clinical [23, 24] studies have demonstrated that superimposed spontaneous breathing during APRV or BIPAP is effective to improve gas exchange, to ventilate the dependent parts of the lung, and to prevent atelectasis.
Combining the wide range of possibilities offered by the BIPAP concept with the original APRV technology, we developed a continuous flow CPAP system equipped with an electromechanical expiratory valve designed to switch between two different CPAP levels on a time-cycled basis (TwinPAP®, StarMed, Mirandola, Modena, Italy). Setting different times for lower and higher CPAP levels determines the rate and duration of mandatory breaths while spontaneous breathing is allowed at all times at the actual PEEP level. In this study, we applied this new device in patients with acute hypoxemic respiratory failure undergoing helmet CPAP. Mandatory breaths were delivered at two different settings as follows: (1) two mandatory breaths per minute to provide periodical hyperinflations (SIGH) [25], and (2) ten mandatory breaths per minute similarly to a commonly used BIPAP setting [21].
The preliminary results of this study were presented at the 2008 meeting of the European Society of Intensive Care Medicine [26].
Materials and methods
Study population
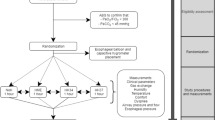
The study population consisted of 21 patients with acute nonhypercapnic hypoxemic respiratory failure admitted to the ICU of San Gerardo Hospital between January 2008 and March 2009. The study protocol was approved by the Institutional Ethical Committee and informed consent was obtained according to the Committee recommendations. Inclusion criteria were treatment with continuous gas flow CPAP delivered through a helmet, onset of the disease <48 h, PaO2/FiO2 < 300 mmHg despite FiO2 ≥ 40% and PEEP ≥ 8 cmH2O, and preserved or normal mental status. Exclusion criteria were cardiogenic pulmonary edema, immediate need for endotracheal intubation, recent ischemic heart disease, chronic obstructive pulmonary disease, known malignant heart rhythm disturbances, hemodynamic instability, analgesia not fully achieved as assessed with numerical rating scale (NRS ≥ 3) [27], burns, trauma, recent head/neck surgery or anatomical abnormalities, or enrollment in other studies.
Instrumentation
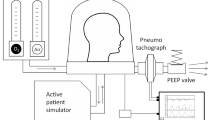
All patients received CPAP via a continuous gas flow system by helmet (CaStar®, StarMed, Mirandola, Modena, Italy). Continuous fresh gas flow was delivered through an adjustable O2/air flowmeter (Harol, San Donato Milanese, Milan, Italy) regulated by the analogue scale of the flowmeter to provide the desired FiO2 with a total flow of 60 l/min. Positive end-expiratory pressure was applied by connecting the expiratory port of the helmet to a TwinPAP® valve system. The TwinPAP® system consists of an electromechanical expiratory valve inserted between two gas outlets (Fig. 1). Standard 22 mm adjustable spring-loaded PEEP valves are applied at the two outlets and regulated to set the higher PEEP level (PEEPhigh) at the outlet proximal to the helmet, and the lower PEEP level (PEEPlow) upstream to the electromechanical valve. When the device is switched off, the electromechanical valve is open to both outlets and gas flows through the PEEPlow valve. When activated, it closes to the PEEPlow outlet directing the gas flow toward the PEEPhighvalve. TwinPAP® is time-cycled by setting the closing (T high) and opening (T low) time (in seconds) of the valve. In vitro testing of the valve for inner resistance of the two inflow-outflow conduits showed a flow-dependent pressure behavior with a prevalent increase in pressure on the PEEPlow side. A gas flow rate of 60 l/min, as applied in this study, causes an increased pressure of 1 cmH2O at the PEEPlow outlet while at 80, 100, and 120 l/min it increases to 2, 3, and 4.5 cmH2O, respectively.
Monitoring
Information about patient clinical history, actual clinical conditions, and ongoing therapy were recorded. Accuracy of delivered FiO2 concentration was checked at the helmet gas inlet by an oximeter monitor (Ohmeda, 4700 OxiCap, Louisville, CO, USA). A small bore (2 mm ID) plastic probe was inserted through the NG tube inlet into the hood to ensure continuous pressure monitoring and recording (the tip placed close to the mouth of the patient). Oxygen peripheral saturation (SpO2), body internal temperature, heart rate (HR), invasive arterial pressure (AP), and central venous pressure (CVP) were continuously monitored. Respiratory rate (RR) was recorded at the end of each step of the protocol.
Study protocol
The study protocol consisted of five steps lasting 60 min each: (1) spontaneous breathing by setting PEEP at 0 cmH2O (SB), (2) CPAP at PEEP 8 cmH2O (CPAPbasal), (3) PEEPlow 8 cmH2O for 25 s and PEEPhigh 25 cmH2O for 5 s (SIGH), (4) PEEPlow 8 cmH2O for 3 s and PEEPhigh 20 cmH2O for 3 s (BIPAP), (5) CPAP with PEEP 8 cmH2O (CPAPfinal). The order of steps 3 and 4 was randomized by a computer-generated random list (Excel, Microsoft®, Redmond, WA, USA).
CPAP, SIGH, and BIPAP were all provided by means of the same continuous gas flow system. No ventilator was used throughout the study. Patients breathed inside the helmet throughout all the steps of the study. Accurate PEEP levels were obtained by regulating the spring-loaded PEEP valves to obtain the desired pressure measurement inside the helmet. The clinically set FiO2 was left unchanged throughout the protocol. All patients were positioned semirecumbent at an angle ≥45° above horizontal to ensure correct pneumatic seal of the helmet. Active humidification was not provided during the protocol.
Statistical analysis
Continuous variables describing hemodynamic and blood gas values were summarized as mean and standard deviation and compared using ANOVA for repeated measurements with Bonferroni correction for multiple comparisons (SPSS 17, Chicago, IL, USA). A p value <0.05 was considered statistically significant.
Results
We enrolled 21 patients (13 males, age 67 ± 17 years, BMI 25.7 ± 3.3). Reasons for instituting helmet CPAP therapy were pneumonia (12), severe sepsis (7), and trauma (2). No major complications (unacceptable leakage, excessive noise, vomiting) were recorded during the protocol. Throughout the procedure patients were questioned for discomfort. No patients reported differences among CPAP, SIGH, and BIPAP in terms of comfort. Some patients noted higher noise during SIGH and BIPAP compared to CPAP. No patient asked for protocol interruption, even when higher pressure steps were applied.
Table 1 reports major clinical variables at different steps of the study. CPAPbasal resulted in a significant increase in PaO2 and PaO2/FiO2 compared to SB (16.5 ± 20%, range −14.8 to 75%, Fig. 2). Application of SIGH or BIPAP further increased PaO2 with respect to CPAPbasal (17 ± 24%, range from −13 to 73% during SIGH, and 25 ± 30.6%, range from −5.8 to 100.7% during BIPAP, Fig. 2). There was no significant difference between SIGH and BIPAP in terms of PaO2.
PaO2/FiO2 ratio at different steps of the study. SB Spontaneous breathing by setting PEEP at 0 cmH2O, CPAP basal CPAP with PEEP 8 cmH2O, SIGH PEEPlow 8 cmH2O for 25 s and PEEPhigh 25 cmH2O for 5 s, BIPAP PEEPlow 8 cmH2O for 3 s and PEEPhigh 20 cmH2O for 3 s, CPAP final CPAP with PEEP 8 cmH2O. *p < 0.05 versus SB, #p < 0.05 versus CPAPbasal
The improvement in PaO2 obtained during SIGH and BIPAP was maintained for at least 1 h of CPAP. Indeed, PaO2 was significantly higher during CPAPfinal compared to CPAPbasal (Fig. 2).
The increase in PaO2 during SIGH or BIPAP compared to CPAPbasal was not correlated with the improvement in PaO2 from SB to CPAPbasal (Fig. 3).
Correlation between the effect of CPAP with respect to spontaneous breathing (expressed as relative changes in PaO2 between CPAPbasal and SB), reported on the abscissa, and the effect of SIGH with respect to CPAP (expressed as relative changes in PaO2 between SIGH and CPAPbasal), reported on the ordinate. We arbitrarily defined a positive PaO2 improvement as any increase in PaO2 greater than 10% (dashed lines). Circles represent patients with PaO2 increase greater than 10% from CPAPbasal to SIGH. Triangles represent patients with PaO2 changes of less than 10% from CPAPbasal to SIGH. Solid symbols represent patients with PaO2 increase greater than 10% from SB to CPAPbasal. Only in patients designated with solid circles (right upper quadrant) were PaO2 increases effected for both CPAP and SIGH. The figure clearly shows that some patients not responding to CPAP may respond to SIGH (empty circles on left upper quadrant)
There were no significant differences between different steps in terms of all other studied variables (Table 1).
Discussion
The main finding of this study is that superimposed, nonsynchronized positive pressure breaths delivered during helmet CPAP in patients with acute hypoxemic respiratory failure improve oxygenation compared to CPAP and spontaneous breathing. Improvement in oxygenation was comparable when delivering two sighs at 25 cmH2O/min or ten breaths at 20 cmH2O/min. The positive effect on oxygenation persisted for at least 1 h after resuming CPAP.
In this study we investigated the potential clinical benefits of a new device, called TwinPAP®, that when mounted on the expiratory limb of any continuous flow CPAP system enables time-cycling between two different CPAP levels. Although the device allows a wide range of time and pressure possibilities, for the purpose of this study we explored only two different settings: administration of 25 cmH2O pressure for 5 s two times per minute, and administration of 20 cmH2O pressure for 3 s ten times per minute. These two different settings correspond to different ventilatory strategies. During SIGH, high inspiratory pressure generating hyperinflation is applied for a longer time but less frequently with the sole purpose of promoting alveolar recruitment. During BIPAP, mandatory breaths are applied at a higher rate but at a lower inspiratory pressure and for shorter time to provide part of ventilatory needs and to improve oxygenation by increasing the mean airway pressure. Choice of pressure and time was mainly dictated by the characteristics of the helmet and by the rate of continuous gas flow. Given the high volume and compliance of the helmet, 5 and 3 s were the minimum times necessary to consistently reach, respectively, 25 and 20 cmH2O at continuous gas flow of 60 l/min. During BIPAP an inspiratory time longer than 3 s would not have been possible given the higher rate of mandatory breaths, and use of pressure higher than 20 cmH2O would have been futile.
Compared to spontaneous breathing or CPAP with 8 cmH2O PEEP, both settings significantly improved oxygenation in patients with acute hypoxic respiratory failure. These results extend to noninvasive ventilation the observation of previous studies performed in mechanically ventilated patients showing improvement of oxygenation during application of sighs in combination with other forms of ventilation [25, 28–31] or during application of BIPAP [20–24].
In this study, the application of CPAP with 8 cmH2O PEEP significantly increased arterial oxygenation compared to spontaneous breathing. However, we found a large patient-to-patient variability in terms of oxygenation response to CPAP application, and in 8 out of 21 patients there was no effect. This may be explained by the heterogeneity of lung disease in our population, with pneumonia being the most represented condition. It is important to observe that there was no correlation between the effect of CPAP over spontaneous breathing with that of SIGH or BIPAP over CPAP, and that five of the eight patients who did not improve during CPAP showed a big improvement in PaO2 after application of SIGH or BIPAP (Fig. 3). Indeed, the potential beneficial effect of SIGH or BIPAP cannot be predicted from the effect of CPAP. We suggest that a stable reopening of atelectatic lung regions promoted by the higher airway pressures applied during SIGH or BIPAP may explain the different effect over CPAP observed in some patients. A stable reopening of atelectasis may also explain why oxygenation improvement was still maintained following discontinuation of SIGH or BIPAP after at least 1 h of CPAP.
Given the design of the study we cannot exclude the possibility that the better oxygenation during CPAPfinal may simply reflect an ongoing improvement over time independent from study treatments. However, given the nature of underlying diseases (patients with cardiogenic pulmonary edema were excluded), we exclude, at least in most patients, that the fast improvement in oxygenation observed in our study could be ascribed to improvement of the disease.
The choice of having basal and final CPAP steps unrandomized rather than randomizing all steps was dictated by the observation in preliminary experience that, after application of SIGH and BIPAP, many patients maintained the improvement in oxygenation for several hours after returning to CPAP. A study design with full randomization of all steps would have required a much larger patient population to demonstrate a significant difference between SIGH or BIPAP and CPAP. Thus, the CPAPbasal serves as basal reference for all successive steps, while CPAPfinal serves to show persistency of recruitment effect after returning to CPAP. In other studies investigating the effect of sighs, the improvement in gas exchange was lost in the majority of patients after sigh discontinuation [25, 28–30]. However, in those studies, sigh pressures ranged from 35 to 45 cmH2O. We speculate that alveoli reopened by such high pressures likely have a stronger tendency to collapse than alveoli reopened by the much lower pressure applied in the present study.
In spite of the higher rate of mandatory breaths administered during BIPAP, the increase in PaO2 was not significantly better than during SIGH. In part, this may be due to the higher pressure applied during SIGH. However, we suggest that in most patients with reversible atelectasis a few breaths per minute are sufficient to obtain a stable improvement in oxygenation. This hypothesis is supported by several studies investigating the effect of sighs in patients with different forms of lung injury [25, 28–31].
We did not observe any significant effect on PaCO2 or respiratory rate during either SIGH or BIPAP. This lack of effect may be considered disappointing, especially during BIPAP, since some degree of ventilatory support is desirable and expected when delivering mandatory breaths in spontaneously breathing patients. However, the present study was not designed to demonstrate the effectiveness of TwinPAP® in providing ventilatory support. First, the studied population consisted of patients with pure oxygenation deficiency but full ventilatory muscle ability. In fact the basal PaCO2 was within the normal range with a tendency of arterial blood pH towards alkalosis. Second, during this study a continuous flow rate of 60 l/min was applied. Such a rate is sufficiently high to grant an effective helmet CPAP system with a minimized risk of CO2 rebreathing, and sufficiently low relative to the flow dependency of the TwinPAP® expiratory valves, thus assuring a safe delivery of pressure inside the helmet. However, given the high compliance and volume of the helmet, at such a flow rate the pressure increase inside the helmet after switching from PEEPlow to PEEPhigh is too slow to provide effective ventilator support. Finally, while it may not be critical to increase alveolar pressure and improve oxygenation, synchronization is crucial for providing effective ventilatory support. This is clearly demonstrated by studies comparing the effectiveness of PSV delivered by helmet with that delivered through a classical face mask [3, 9, 10]. In fact, helmet PSV proves to be less effective than face mask PSV in unloading patient effort and decreasing PaCO2, while the benefit in terms of oxygenation is similar with both interfaces.
This is the first clinical application of the TwinPAP® system. No technical problems were encountered throughout the study, and the device proved to be safe. Due to the inner flow resistance of the lower PEEP level outlet and depending on the characteristics of the mechanical PEEP valves connected to the TwinPAP® expiratory outlets, the pressure inside the helmet may be unacceptably higher than the intended one. In order to ensure full patient safety during application of the device, we recommend the use of an adjustable gas-flow delivery system and low resistance mechanical PEEP valves. However, the possibility of directly measuring the pressure inside the helmet allows pressure to be fully controlled throughout mandatory respiratory cycles.
The TwinPAP® system is easy to set up and does not add additional settings complications to the commonly used helmet CPAP systems. We suggest that the TwinPAP® system may be safely applied in all settings were helmet CPAP is currently used [4, 6–8], even in patients in whom CPAP failed to improve oxygenation.
In conclusion, superimposed, nonsynchronized positive pressure breaths delivered during helmet CPAP by means of the TwinPAP® system may improve oxygenation in patients with acute hypoxemic respiratory failure, even at an administration rate as low as two breaths per minute. The system is safe and may be theoretically applied in all clinical settings where helmet CPAP is commonly used.
References
Esteban A, Ferguson ND, Meade MO, Frutos-Vivar F, Apezteguia C, Brochard L, Raymondos K, Nin N, Hurtado J, Tomicic V, González M, Elizalde J, Nightingale P, Abroug F, Pelosi P, Arabi Y, Moreno R, Jibaja M, D’Empaire G, Sandi F, Matamis D, Montañez AM, Anzueto A, Group VENTILA (2008) Evolution of mechanical ventilation in response to clinical research. Am J Respir Crit Care Med 177:170–177
Antonelli M, Conti G, Pelosi P, Gregoretti C, Pennisi MA, Costa R, Severgnini P, Chiaranda M, Proietti R (2002) New treatment of acute hypoxemic respiratory failure: noninvasive pressure support ventilation delivered by helmet––a pilot controlled trial. Crit Care Med 30:602–608
Antonelli M, Pennisi MA, Pelosi P, Gregoretti C, Squadrone V, Rocco M, Cecchini L, Chiumello D, Severgnini P, Proietti R, Navalesi P, Conti G (2004) Noninvasive positive pressure ventilation using a helmet in patients with acute exacerbation of chronic obstructive pulmonary disease: a feasibility study. Anesthesiology 100:16–24
Principi T, Pantanetti S, Catani F, Elisei D, Gabbanelli V, Pelaia P, Leoni P (2004) Noninvasive continuous positive airway pressure delivered by helmet in hematological malignancy patients with hypoxemic acute respiratory failure. Intensive Care Med 30:147–150
Patroniti N, Foti G, Manfio A, Coppo A, Bellani G, Pesenti A (2003) Head helmet versus face mask for non-invasive continuous positive airway pressure: a physiological study. Intensive Care Med 29:1680–1687
Tonnelier JM, Prat G, Nowak E, Goetghebeur D, Renault A, Boles JM, L’her E (2003) Noninvasive continuous positive airway pressure ventilation using a new helmet interface: a case-control prospective pilot study. Intensive Care Med 29:2077–2080
Foti G, Sangalli F, Berra L, Sironi S, Cazzaniga M, Rossi GP, Bellani G, Pesenti A (2009) Is helmet CPAP first line pre-hospital treatment of presumed severe acute pulmonary edema? Intensive Care Med 35:656–662
Squadrone V, Coha M, Cerutti E, Schellino MM, Biolino P, Occella P, Belloni G, Vilianis G, Fiore G, Cavallo F, Ranieri VM, Piedmont Intensive Care Units Network (PICUN) (2005) Continuous positive airway pressure for treatment of postoperative hypoxemia: a randomized controlled trial. JAMA 293:589–595
Navalesi P, Costa R, Ceriana P, Carlucci A, Prinianakis G, Antonelli M, Nava S, Conti G (2007) Non-invasive ventilation in chronic obstructive pulmonary disease patients: helmet versus facial mask. Intensive Care Med 33:74–81
Rocco M, Dell’Utri D, Morelli A, Spadetta G, Conti G, Antonelli M, Pietropaoli P (2004) Noninvasive ventilation by helmet or face mask in immunocompromised patients: a case-control study. Chest 126:1508–1515
Vargas F, Thille A, Lyazidi A, Campo FR, Brochard L (2009) Helmet with specific settings versus facemask for noninvasive ventilation. Crit Care Med 37:1921–1928
Racca F, Appendini L, Gregoretti C, Stra E, Patessio A, Donner CF, Ranieri VM (2005) Effectiveness of mask and helmet interfaces to deliver noninvasive ventilation in a human model of resistive breathing. J Appl Physiol 99:1262–1271
Racca F, Appendini L, Berta G, Barberis L, Vittone F, Gregoretti C, Ferreyra G, Urbino R, Ranieri VM (2009) Helmet ventilation for acute respiratory failure and nasal skin breakdown in neuromuscular disorders. Anesth Analg 109:164–167
Taccone P, Hess D, Caironi P, Bigatello L (2004) Continuous positive airway pressure delivered with a “helmet”: effects on carbon dioxide rebreathing. Crit Care Med 32:2090–2096
Mojoli F, Iotti GA, Gerletti M, Lucarini C, Braschi A (2008) Carbon dioxide rebreathing during non-invasive ventilation delivered by helmet: a bench study. Intensive Care Med 34:1454–1460
Costa R, Navalesi P, Antonelli M, Cavaliere F, Craba A, Proietti R, Conti G (2005) Physiologic evaluation of different levels of assistance during noninvasive ventilation delivered through a helmet. Chest 128:2984–2990
Nava S, Navalesi P (2009) Helmet to deliver noninvasive ventilation: “handle with care”. Crit Care Med 37:2111–2113
Downs JB, Stock MC (1987) Airway pressure release ventilation: a new concept in ventilatory support. Crit Care Med 15:459–461
Stock MC, Downs JB, Frolicher DA (1987) Airway pressure release ventilation. Crit Care Med 15:462–463
Baum M, Benzer H, Putensen C, Koller W, Putz G (1989) Biphasic positive airway pressure (BIPAP)––a new form of augmented ventilation. Anaesthesist 38:452–458
Hörmann C, Baum M, Putensen C, Mutz NJ, Benzer H (1994) Biphasic positive airway pressure (BIPAP)––a new mode of ventilatory support. Eur J Anaesthesiol 11:37–42
Putensen C, Muders T, Varelmann D, Wrigge H (2006) The impact of spontaneous breathing during mechanical ventilation. Curr Opin Crit Care 12:13–18
Hering R, Zinserling J, Wrigge H, Varelmann D, Berg A, Kreyer S, Putensen C (2005) Effects of spontaneous breathing during airway pressure release ventilation on respiratory work and muscle blood flow in experimental lung injury. Chest 128:2991–2998
Putensen C, Mutz NJ, Putensen-Himmer G, Zinserling J (1999) Spontaneous breathing during ventilatory support improves ventilation-perfusion distributions in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 159:1241–1248
Patroniti N, Foti G, Cortinovis B, Maggioni E, Bigatello LM, Cereda M, Pesenti A (2002) Sigh improves gas exchange and lung volume in patients with acute respiratory distress syndrome undergoing pressure support ventilation. Anesthesiology 96:788–794
Isgrò S, Vergnano B, Foti G, Redaelli S, Mangili P, Bellandi M, Castagna L, Bellani G, Pesenti A, Patroniti N (2008) Helmet for ARF in ICU: CPAP versus periodical application of two pressure levels. Intensive Care Med 34(S1):S88
Breivik H, Borchgrevink PC, Allen SM, Rosseland LA, Romundstad L, Hals EK, Kvarstein G, Stubhaug A (2008) Assessment of pain. Br J Anaesth 101:17–24
Pelosi P, Cadringher P, Bottino N, Panigada M, Carrieri F, Riva E, Lissoni A, Gattinoni L (1999) Sigh in acute respiratory distress syndrome. Am J Respir Crit Care Med 159:872–880
Foti G, Cereda M, Sparacino ME, De Marchi L, Villa F, Pesenti A (2000) Effects of periodic lung recruitment maneuvers on gas exchange and respiratory mechanics in mechanically ventilated acute respiratory distress syndrome (ARDS) patients. Intensive Care Med 26:501–507
Pelosi P, Chiumello D, Calvi E, Taccone P, Bottino N, Panigada M, Cadringher P, Gattinoni L (2001) Effects of different continuous positive airway pressure devices and periodic hyperinflations on respiratory function. Crit Care Med 29:1683–1689
Pelosi P, Bottino N, Chiumello D, Caironi P, Panigada M, Gamberoni C, Colombo G, Bigatello LM, Gattinoni L (2002) Sigh in supine and prone position during acute respiratory distress syndrome. Am J Respir Crit Care Med 167:521–527
Acknowledgments
Starmed provided a research grant to Milano-Bicocca University for the purpose of the study. TwinPAPs®, helmets, and threshold valves were supplied by Starmed.
Conflict of interest statement
N. Patroniti and A. Pesenti patented an invention related to TwinPAP® (European patent application EP 1 790 369 A1). The license for this patent belongs to Starmed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Isgrò, S., Zanella, A., Sala, C. et al. Continuous flow biphasic positive airway pressure by helmet in patients with acute hypoxic respiratory failure: effect on oxygenation. Intensive Care Med 36, 1688–1694 (2010). https://doi.org/10.1007/s00134-010-1925-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-1925-2