Abstract
Purpose
To identify the risk factors of ventilator-associated pneumonia (VAP) due to difficult-to-treat (DTT) bacteria (i.e., Pseudomonas aeruginosa, Acinetobacter baumannii and oxacillin-resistant Staphylococcus aureus), and to assess the rate and the causes of inappropriateness of empirical antimicrobial therapy.
Methods
In an intensive care unit of a university hospital, patients with VAP were empirically treated with antibiotics without activity against DTT bacteria if the patients had no prior hospitalization or prior administration of antibiotics, according to local guidelines.
Results
Overall, the empirical antimicrobial therapy was appropriate in 190 (87%) out of 218 patients with VAP. Fifty (23%) patients developed problems due to DTT bacteria. The risk factors for VAP due to DTT bacteria were shock state, prior antimicrobial therapy, prior stay in long-term care facilities and late-onset VAP. Empirical antimicrobial therapy was inappropriate in 20 (40%) patients with VAP due to DTT bacteria and 8 (5%) patients with VAP due to non-DTT (P = 0.001). Guidelines violations (nine patients), bacteria not included in antibiotic spectrum (eight patients) and bacterial resistance (three patients) were the causes of inappropriateness in case of DTT bacteria.
Conclusion
Despite the abundant information for the treatment of VAP and the establishment of guidelines, too many patients with DTT bacteria received inappropriate antimicrobial therapy. Since 45% of the cases are related to non-adherence to the local protocol, there is room for improvement by implementing educational programs. Also, since DTT bacteria are found in 23% of late-onset VAP, empirical antibiotic treatment should be directed against these pathogens.
Similar content being viewed by others
Introduction
The incidence of ventilator-associated pneumonia (VAP) in intensive care units (ICU) ranges from 5 to around 30% [1, 2]. Such infections prolong hospital stay and may contribute to ICU mortality [3–5]. Despite guidelines on the management of VAP [3, 4], recent reports emphasize the emergence of difficult-to-treat bacteria [oxacillin-resistant Staphylococcus aureus (ORSA), Pseudomonas aeruginosa and Acinetobacter baumannii] [6–9]. Several studies suggested that these microorganisms are important determinants of patient survival [10, 11]. The appropriateness of empirical antimicrobial therapy for VAP is a key determinant for patient outcome [12–14].
We performed a clinical investigation with the following objectives: (1) to identify the risk factors for developing VAP due to difficult-to-treat bacteria compared with non-difficult-to-treat bacteria, (2) to assess the rate of inappropriateness of empirical antimicrobial therapy according to local guidelines established by the local ICU staff and the hospital microbiologists, and (3) to identify the causes of inappropriateness.
Materials and methods
The study was conducted in a 16-bed ICU in an 800-bed university hospital (Hôpital Nord, Marseille, France). Informed consent and approval by the Ethics Committee were waived due to the observational nature of the study. During a 6-year period (January 2001–January 2007), all patients admitted to the ICU requiring tracheal intubation and mechanical ventilation for more than 48 h were eligible for inclusion.
Patients
Patients with VAP were retrospectively included in the cohort. The diagnosis of VAP was established when the following criteria were fulfilled: (1) purulent bronchial sputum, (2) body temperature >38 or <36°C, (3) white blood cells >10,000 or <4,000 per mm3, (4) chest radiograph showing new or progressive infiltrates, (5) presence of at least one microorganism at a concentration of ≥104 colony-forming units (cfu) per ml in bronchoalveolar lavage or 106 cfu/ml in tracheal aspirates. We excluded episodes in which the final diagnosis was rejected or undetermined due to the presence of an alternative cause or in which no agreement on diagnosis was reached. Senior intensivists and microbiologists rejected or determined the final diagnosis of VAP.
The unit is set up on a closed organizational format, with two weekly staff meetings, including a meeting with the microbiology physicians. The plan of care was decided during the daily round by the attending physician. The duration of prior antibiotic exposure was recorded as a dichotomous variable with a threshold to 3 days to discriminate antimicrobial prophylaxis for trauma patients (that can be prescribed up to 2 days) and prior use of antimicrobial therapy. Prior broad-spectrum antimicrobial therapy (third-generation cephalosporin, amoxicillin–clavulanate association, fluoroquinolone and antipseudomonal antibiotics) before VAP onset was regrouped to constitute a new variable. Stress-ulcer prophylaxis was used in patients with a past medical history significant for ulcer, as described elsewhere [15]. Patients received early enteral nutrition if possible. They underwent mechanical ventilation with the ventilator set in volume-controlled mode. Heat and moisture exchanger bacterial filters (Gibeck, Stockholm, Sweden) were inserted between the endotracheal tube and the ventilator circuit except in patients with acute respiratory distress syndrome. Patients were placed in the semi-recumbent position. Trauma patients received selective digestive decontamination consisting of polymyxin E, gentamicin and amphotericin B, as described elsewhere [16]. For the first 3 days, systemic cefazolin (1 g × 3 daily) was given to all trauma patients. Selective digestive decontamination was started on the day of admission and was continued until the patients were weaned from mechanical ventilation.
Empirical antimicrobial therapy
Antimicrobial therapy was administered according to our local written guidelines, as described elsewhere [16]. Empirical antimicrobial therapy was initiated just after microbiological sample collection. Ceftriaxone or amoxicillin–clavulanate was used in the patients with early onset VAP (<5 days) without recent prior hospitalization, including long-term care facilities (within 30 days), or prior antibiotic treatment (within 15 days). If intracellular infection was suspected, macrolides were added at the discretion of the attending physician. Gentamicin was combined with a beta-lactam in patients with severe sepsis. Patients who developed late-onset VAP (≥5 days) without prior hospitalization, prior administration of antibiotics except for surgical prophylaxis and selective digestive decontamination, and signs of severity defined by the occurrence of acute respiratory distress syndrome or severe sepsis or septic shock, were treated with ceftriaxone (limited spectrum). In all other cases beta-lactams with activity against P. aeruginosa (piperacillin–tazobactam or ceftazidime) were used at the discretion of the attending physicians (antipseudomonal antibiotics). Carbapenems were kept as last-line therapy. Amikacin or levofloxacin was added when criteria for severe sepsis or septic shock were diagnosed. Vancomycin or linezolid (in case of renal failure) was prescribed when ORSA was suspected: previous colonization in patient’s microbiological samples, medical patient with prior broad-spectrum antimicrobial therapy over the previous 2 weeks and gram stain with gram-positive cocci. Empirical antimicrobial therapy was systematically reassessed from the moment the microbiological results were available (i.e., on days 2–3). Microbiological findings were used to determine whether the empirical treatment targeted the identified bacteria. The clinical status of patients was evaluated taking into account the evolution of prior organ dysfunction and plasma lactate levels. According to the antimicrobial susceptibility of the identified bacteria and clinical progress, a decision was made to perform escalation, de-escalation or maintain the initial treatment.
Definitions
Details on the definitions of “difficult-to-treat bacteria,” “antipseudomonal antibiotics,” “limited-spectrum antibiotics,” “inappropriate empirical antimicrobial therapy” [17], “clinical resolution,” “de-escalation” and “escalation” are provided in the Electronic Supplementary Files. The Clinical Pulmonary Infection Score (CPIS) was used to assess the clinical response of patients to treatment [18]. Death was related to VAP if the pulmonary infection was a contributing factor to death in patients with co-morbidity [16].
Microbiology
Collections and cultures of microbiologic samples were performed as described elsewhere [16]. Detailed description of microbiologic practices is reported in Electronic Supplementary Files.
Variables
The following variables were recorded at ICU admission: age, gender, comorbidities, prior hospitalization within 30 days, case mix, Glasgow Coma Scale score, prior antibiotic treatment within 15 days and Simplified Acute Physiology Score (SAPS) II [19]. The following variables were recorded on the day of sample collection: body temperature, white blood cell count, PaO2/FIO2 ratio and lactate plasma level >2 mmol/l. The duration of mechanical ventilation and length of ICU stay were also recorded.
Statistical analysis
A descriptive analysis was performed. Statistical calculations were performed using SPSS software version 15.0. The distribution of continuous variables was checked; if the distribution was normal, data were expressed as mean values ± standard deviation (SD). For ordinal variables or if the distribution was not normally distributed, data were expressed as median values with interquartile range (quartile 25–75%). For categorical variables, percentages were computed. Comparisons of quantitative values of dichotomous variables were performed with the Student’s t test or Mann–Whitney U test for non-normally distributed variables. Comparisons of percentages were performed with Fisher’s exact test. A multidimensional analysis by logistic regression using the forward Wald stepwise model was used to determine which items were the most pertinent in the predictive model. After univariate analysis, values were included when significance was <0.25 or in the case of clinical relevance. Calibration was the difference between the predicted and observed value by stratification; the Hosmer–Lemeshow test was used [20]. A p value >0.1 indicates good calibration. Discrimination is the aptitude to distinguish the true positive and true negative among the patients; the area under the receiver-operating characteristic curve was used [21]. For each decision criterion, sensitivity and specificity were computed. Briefly, an area up to 50% indicates a random phenomenon. A large area with a low p value indicates a good discrimination. Predictive positive and negative value and percentage of appropriately classified patients by the model were also computed. All comparisons were two tailed, and a p value of <0.05 was required to exclude the null hypothesis.
Results
Among 6,172 admissions during the 6-year study period, 218 patients requiring tracheal intubation for more than 48 h developed VAP (Table 1). The bacteria responsible for VAP are listed in Table 2. Fifty patients developed VAP due to difficult-to-treat bacteria (Table 3). Seventy-six (35%) patients developed early-onset VAP. Among them, nine (4%) patients had risk VAP due to difficult-to-treat bacteria. One hundred forty-two (65%) patients developed late-onset VAP. Among them, 41 (19%) patients had VAP due to difficult-to-treat bacteria.
Risk factors for difficult-to-treat bacteria
The variables identified by univariate analysis included in the stepwise logistic regression model were: medical patients, smoking status, medication (corticosteroids, anti-acids), recurrent tracheal intubations, tracheotomy, concomitant shock, prior exposure to antibiotics within 15 days, prior stay in hospital or long-term care facilities within 30 days, and late-onset VAP. Prior hospitalization was found in 23 (46%) patients with VAP due to difficult-to-treat bacteria, as compared with 27 (16%) patients with VAP due to non-difficult-to-treat bacteria (p = 0.001). Forty-two (84%) patients with VAP due to difficult-to-treat bacteria were exposed to antimicrobial therapy within the previous 2 weeks, as compared with 114 (68%) patients with VAP due to non-difficult-to-treat bacteria (p = 0.001). Twenty-five (50%) patients with VAP due to difficult-to-treat bacteria and 25 (15%) patients with VAP due to non-difficult-to-treat bacteria received prior broad-spectrum antimicrobial therapy (p < 0.001). Among 66 medical patients, 21 (42%) patients developed VAP due to difficult-to-treat bacteria, as compared with 45 (68%) patients with VAP due to non-difficult-to-treat bacteria (p = 0.04). The incidence of VAP with difficult-to-treat bacteria (n = 22; 44%) and non-difficult-to-treat bacteria (n = 97; 58%) was similar in trauma patients treated with selective digestive decontamination.
The independent risk factors for acquiring VAP due to difficult-to-treat bacteria were concomitant shock state, prior antimicrobial therapy for longer than 3 days, prior hospitalization or stay in long-term care facilities, and late-onset of VAP (Table 4). The discrimination of the model was acceptable with an area under the curve at 0.78 (0.71–0.87) (p < 0.001, R² = 0.29). One hundred sixty-eight (77%) patients were adequately classified with a cutoff providing a sensitivity of 54% and specificity of 83%.
Rate and causes of inappropriateness of the empirical antimicrobial therapy in patients with difficult-to-treat bacteria
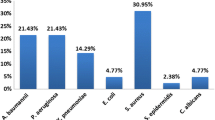
Empirical antimicrobial therapy was inappropriate in 20 (40%) patients with VAP due to difficult-to-treat bacteria and in 8 (5%) patients with VAP due to non-difficult-to-treat bacteria (p < 0.001). Thirty-two (30%) patients with VAP due to difficult-to-treat bacteria were treated with antipseudomonal antibiotics, compared with 31 (18%) patients with VAP due to non-difficult-to-treat bacteria (p < 0.001). The causes of inappropriateness are reported in Fig. 1. In nine (45%) cases, guideline violation was responsible for inappropriateness in case of difficult-to-treat bacteria. Especially, eight patients with late-onset VAP and risk factors for difficult-to-treat bacteria were treated with non-antipseudomonal antibiotics. In the other cases, the causes were linked to the presence of multidrug-resistant bacteria, despite guidelines being strictly followed.
Outcomes
Empirical antimicrobial therapy was de-escalated in 108 (50%) patients on day 3 (2–5), including 27 (54%) patients with difficult-to-treat bacteria and 81 (48%) with other bacteria (p = 0.6). The antimicrobial therapy was administered for 10 (7–15) days in the patients with VAP due to difficult-to-treat bacteria, compared with 8 (7–10) days for those with VAP due to non-difficult-to-treat bacteria (p = 0.03). The duration of mechanical ventilation was prolonged in the patients with VAP due to difficult-to-treat bacteria [14 (9–27) vs. 11 (6–18) days, p = 0.01].
Death was attributable to VAP in 7 (14%) patients with VAP due to difficult-to-treat bacteria and 22 (13%) patients with VAP due to non-difficult-to-treat bacteria (p = 0.7). With respect to resolution of VAP, there was no significant difference between patients with VAP due to difficult-to-treat bacteria and those with VAP due to non-difficult-to-treat bacteria (Table 3). Recovery was delayed in the patients with VAP due to difficult-to-treat bacteria, compared with those with VAP due to non-difficult-to-treat bacteria [8 (5–12) days vs. 7 (5–9) days, p = 0.02]. After adjustment for the appropriateness of antimicrobial therapy, 24 (80%) out of 30 patients with VAP due to difficult-to-treat bacteria recovered, as compared with 135 (84%) out of 160 patients with VAP due to non-difficult-to-treat bacteria (p = 0.6) (Fig. 2).
Discussion
In the present study, the independent risk factors of VAP due to difficult-to-treat bacteria were previous antibiotic exposure (less than 2 weeks), recent hospitalization over the last month, concomitant shock state and late-onset of VAP. Empirical antimicrobial therapy was inappropriate in 40% of the patients with VAP due to difficult-to-treat bacteria and 5% in case of non-difficult-to-treat bacteria. In almost half of the cases, inappropriate empirical antimicrobial therapy for difficult-to-treat bacteria was due to non-adherence to the local guidelines.
For most of the patients with treatment failure, this was explained by the prescription of ceftriaxone despite the presence of risk factors for difficult-to-treat bacteria. The guidelines clearly state that in such patients, an antipseudomonal agent should have been prescribed. Thus, there is room for improvement, for instance by establishing educational programs and updating our guidelines according to local microbiologic data. The use of ceftriaxone is now excluded for late-onset VAP since late-onset by itself is an independent risk factor for difficult-to-treat bacteria. The other failures are due to the intrinsic limitations of any guidelines. Indeed, no guidelines can cover all potential pathogens responsible for VAP, whether or not due to difficult-to-treat bacteria.
In agreement with previous studies, we found that prior medical history is a major determinant for the choice of the empirical antimicrobial therapy [16, 17, 22–24]. Recent hospitalization and prior use of antibiotics are key factors for selecting patients at high risk of difficult-to-treat bacterial carriage. The occurrence of concomitant septic shock was associated with an increased rate of VAP due to difficult-to-treat bacteria. The incidence of lung infection-related septic shock has increased during the last decade [25]. In parallel, the incidence of septic shock due to multidrug-resistant pathogens is also increasing [25]. Thus, antipseudomonal antibiotics should probably be used in most patients with septic shock due to VAP. Finally, the time of onset of VAP remains an important determinant for the presence of difficult-to-treat bacteria. In line with the literature, we found that a period of 4 days before VAP occurrence was an independent risk factor of difficult-to-treat bacteria [24, 26].
Several studies showed that the appropriateness of empirical antimicrobial therapy for VAP is associated with the rate of mortality [13, 14, 22]. However, this relationship remains unclear in the cases of difficult-to-treat bacteria. The present study was not designed to assess this issue specifically and probably underpowered in that regard.
To minimize the risk of emergence of antibiotic resistance, our guidelines demand a reappraisal of initial antimicrobial therapy as soon as the bacteria responsible for VAP are identified. Hence, the empirical antimicrobial therapy was deescalated in 27 (54%) of the patients with difficult-to-treat bacteria. In agreement with our previous findings, we showed here that de-escalation is possible in a large number of patients with VAP, even when infection is caused by difficult-to-treat bacteria [16]. This finding contrasts to that obtained in a prior study, since only three percent of treatments were deescalated in patients with difficult-to-treat bacteria [27]. In order to reduce the emergence of multidrug-resistant bacteria, the duration of antimicrobial therapy should not exceed 8 days [28]. However, this recommendation remains unclear in the cases of non-fermenting gram-negative bacteria [28]. Our data show, within the study period, VAP due to difficult-to-treat bacteria were treated for around 10 days.
Our study has several limitations. This was a single-center study. Our findings may be attributed to institution-specific variables. Thereby, it raises the possibility of institutional bias either in patient selection or in other institutional practices. Thus, one can only draw very limited conclusions about guideline performance and rates of guideline non-adherence since institutional factors likely play a large role in physician prescribing culture, bacterial ecology, antibiotic resistance rates and patient severity of illness. Most of our patients were trauma patients (55%) who received antimicrobial prophylaxis due to various procedures and selective digestive decontamination. The impact of 3 days of systemic antibiotic on commensal flora remains matter of debate [29]. However, in a prior study, we showed the weak ecological impact of prolonged use of selective digestive decontamination in our ICU [30]. Finally, we found that the duration of mechanical ventilation was longer for patients with difficult-to-treat bacteria than in those with non-difficult-to-treat bacteria. This is still true after adjustment for the appropriateness of empirical antibiotic treatment. One cannot exclude that this finding was related to co-morbidities, although the severity score was higher in the patients with non-difficult-to-treat bacteria than in those with difficult-to-treat bacteria.
In conclusion, our empirical antibiotic treatment was appropriate in 87% of the patients with VAP. In this population, prior hospitalization, late-onset VAP, prior antimicrobial therapy and concomitant shock increase the risk factors for VAP due to difficult-to-treat bacteria. Empirical antimicrobial therapy was inappropriate in 40% of the patients with VAP due to difficult-to-treat bacteria. Guideline violation occurred in half of the cases of inappropriateness. Thus, it seems important to implement “bundle of care” with feedback information. However, inappropriateness due to non-adherence to guidelines represented only 6% of the treated patients. In other studies, compliance with guidelines was far from this level [31]. On the other hand, another way to improve appropriateness is to update our guidelines in accordance with microbiologic data and recommend broad-spectrum antimicrobial therapy for late-onset VAP.
References
Kollef MH (1993) Ventilator-associated pneumonia: a multivariate analysis. JAMA 270:1965–1970
Torres A, Aznar R, Gatell JM, Jiménez P, Gonzalez J, Ferre A, Celis R, Rodriguez-Roisin R (1990) Incidence, risk, and prognosis factors of nosocomial pneumonia in mechanically ventilated patients. Am Rev Respir Dis 142:523–528
Craven DE, Kunches LM, Lichtenberg DA, Kollisch NR, Barry MA, Heeren TC, McCabe WR (1998) Nosocomial infection and fatality in medical and surgical intensive care unit patients. Arch Intern Med 148:1161–1168
Craig CP, Connelly S (1984) Effect of intensive care unit nosocomial pneumonia on duration of stay and mortality. Am J Infect Control 12:233–238
Fagon JY, Chastre J, Vuagnat A, Trouillet JL, Novara A, Gibert C (1996) Nosocomial pneumonia and mortality among patients in intensive care units. JAMA 275:866–869
American Thoracic Society (2005) Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171:388–416
Torres A, Ewig S, Lode H, Carlet J, For the European HAP working group (2009) Defining, treating and preventing hospital acquired pneumonia: European perspective. Intensive Care Med 35:9–29
Schaberg DR, Culver DH, Gaynes RP (1991) Major trends in the microbial etiology of nosocomial infection. Am J Med Suppl 3B:72S–75S
Emori TG, Gaynes RP (1993) An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev 6:428–442
Spencer RC (1996) Predominant pathogens found in the European prevalence of infection in intensive care study. Eur J Clin Microbiol Infect Dis 15:281–285
Stevens RM, Teres D, Skillman JJ, Feingold DS (1974) Pneumonia in an intensive care unit. Arch Intern Med 134:106–111
Fagon JY, Chastre J, Domart Y, Trouillet JL, Pierre J, Darne C, Gibert C (1989) Nosocomial pneumonia in patients receiving continuous mechanical ventilation: prospective analysis of 52 episodes with use of a protected specimen brush and quantitative culture techniques. Am Rev Respir Dis 139:877–884
Ibrahim EH, Ward S, Sherman G, Schaiff R, Fraser VJ, Kollef MH (2001) Experience with a clinical guideline for the treatment of ventilator-associated pneumonia. Crit Care Med 29:1109–1115
Leroy O, Meybeck A, d’ Escrivan T, Devos P, Kipnis E, Georges H (2003) Impact of appropriateness of initial antimicrobial therapy on the prognosis of patients with ventilator-associated pneumonia. Intensive Care Med 29:2170–2173
Faisy C, Guerot E, Diehl JL, Iftimovci E, Fagon JY (2003) Clinically significant gastrointestinal bleeding in critically ill patients with and without stress ulcer prophylaxis. Intensive Care Med 29:1306–1313
Leone M, Garcin F, Bouvenot J, Boyadjev I, Visintini P, Albanèse J, Martin C (2007) Ventilator-associated pneumonia: breaking the vicious circle of antibiotic overuse. Crit Care Med 35:379–385
Kollef MH, Sherman G, Ward S, Prentice D, Schaiff R, Huey W, Fraser VJ (1999) Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 115:462–474
Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew PD, Suter PM (1991) Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis 143:1121–1129
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Lemeshow S, Hosmer DW Jr (1982) A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol 115:92–106
Hanley JA, McNeil BJ (1982) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143:29–36
Dupont H, Mentec H, Sollet JP, Bleichner G (2001) Impact of appropriateness of initial antibiotic therapy on the outcome of ventilator-associated pneumonia. Intensive Care Med 27:355–362
Kollef MH, Silver P, Murphy DM, Trovillion E (1995) The effect of late-onset ventilator-associated pneumonia in determining patient mortality. Chest 108:1655–1662
Rello J, Allegri C, Rodriguez A, Vidaur L, Sirgo G, Gomez F, Agbaht K, Pobo A, Diaz E (2006) Risk factors for ventilator-associated pneumonia by Pseudomonas aeruginosa in presence if recent antibiotic exposure. Anesthesiology 105:709–714
Annane D, Aergerter P, Jars-Guincestre MC, Guidet B, CUB-Réa Network (2003) Current epidemiology of septic shock: the CUB-Réa Network. Am J Respir Crit Care Med 168:165–172
Rello J, Sa-Borges M, Correa H, Leal SR, Baraibar J (1999) Variations in etiology of ventilator-associated pneumonia across four treatment sites. Am J Respir Crit Care Med 160:608–613
Rello J, Vidaur L, Sandiumenge A, Rodriguez A, Gualis B, Boque C, Diaz E (2004) Deescalation therapy in ventilator-associated pneumonia. Crit Care Med 32:2183–2190
Chastre J, Wolff M, Fagon JY, Chevret S, Thoma F, Wermet D, Clementi E, Gonzalez J, Jusserand D, Asfar P, Perrin D, Fieux F, Aubas S, PneumA Trial Group (2003) Comparison of 8 vs. 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA 290:2588–2598
El Solh AA, Pietrantoni C, Bhat A, Bhora M, Berbary E (2004) Indicators of potentially drug-resistant bacteria in severe nursing home-acquired pneumonia. Clin Infect Dis 39:474–480
Leone M, Albanese J, Antonini F, Nguyen-Michel A, Martin C (2003) Long-term (6-year) effect of selective digestive decontamination on antimicrobial resistance in intensive care, multiple-trauma patients. Crit Care Med 31:2090–2095
Shorr AF, Bodi M, Rodriguez A, Sole-Violan J, Garnacho-Montero J, Rello J, CAPUCI Study Investigators (2006) Impact of antibiotic guideline compliance on duration of mechanical ventilation in critically ill patients with community-acquired pneumonia. Chest 130:93–100
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Garcin, F., Leone, M., Antonini, F. et al. Non-adherence to guidelines: an avoidable cause of failure of empirical antimicrobial therapy in the presence of difficult-to-treat bacteria. Intensive Care Med 36, 75–82 (2010). https://doi.org/10.1007/s00134-009-1660-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-009-1660-8