Abstract
Objective
To investigate tidal volume (V T) and leak measurements during continuous positive airway pressure (CPAP) in neonates using a commercial ventilatory device equipped with a flow sensor at the Y-piece.
Design
Randomized cross-over trial.
Setting
Neonatal intensive care unit level III.
Patients
Thirty-two infants, median (range) birth weight 1,435 (710–2,730) g, gestational age 30 (24–38) weeks.
Interventions
During nasopharyngeal CPAP, leak and V T were measured with and without occlusion of the contralateral nostril using the Leoni ventilator (Heinen & Löwenstein, Germany) and a recently developed algorithm to correct measured V T in the presence of leaks. The measuring range of the Leoni is limited to leaks <90%.
Main results
Analyzable measurements with leaks <90% could be obtained in 12.5% of the patients with open nostril, and in 65.6% with occluded nostril. Calculated leak flow after nostril occlusion was 23 (3–77) ml min−1 with closed mouth. Leak flow increased significantly if mouth was opened (548 (0–1,394) ml min−1, p < 0.001), but was probably even higher where leaks exceeded 90%. Mean expiratory volume ± SD was 5.8 ± 1.3 ml kg−1 (corrected V T 5.9 ± 1.2 ml kg−1) for leaks <20%, and 3.7 ± 1.4 ml kg−1 (corrected V T 5.8 ± 2.2 ml kg−1) for leaks between 20 and 69%.
Conclusions
Leak and corrected V T could be determined in the presence of leaks of up to 69%, but leaks during CPAP often exceeded the measuring range. Reliable volume and leak monitoring was not possible with the tested equipment during nasopharyngeal CPAP. Advanced equipment is necessary to further investigate the effects of leaks on neonatal CPAP therapy.
Similar content being viewed by others
Introduction
Continuous positive airway pressure (CPAP) is increasingly used to treat neonatal breathing disorders, as it appears to reduce the risk of adverse outcomes associated with intubation and mechanical ventilation [1–6]. A variety of commercial CPAP systems are available today, and clinicians can choose from a large range of CPAP interfaces that differ with regard to resistance, dead space and proneness to air leakages [7, 8].
As yet, little research has been carried out into air leakages during CPAP in newborns. However, investigations in adults have shown that leaks are a key issue during CPAP, as they have a substantial influence on the efficacy and side effects of CPAP therapy [9–12]. Leak monitoring during CPAP might facilitate the selection of more appropriate CPAP settings and interfaces [13, 14], but it is technically difficult and depends on the CPAP system used.
In adults, modern CPAP devices adjust the background flow to maintain a constant CPAP level. These devices allow quantitative leak measurements, either by a built-in pneumotachograph of the flow driver or by measuring the rotation speed of the flow generator [15].
In neonates, airflow and leak measurements during CPAP are more intricate than in adults owing to small signal amplitudes, higher impact of apparatus dead space and reduced signal-to-noise ratio. Moreover, currently preferred CPAP interfaces such as binasal prongs and nasopharyngeal tubes [16] predispose neonates to significant nose and mouth leaks, which further complicate flow-signal analysis. To circumvent these problems, most ventilatory measurements during nasal CPAP were performed using indirect methods such as respiratory inductive plethysmography [17, 18]. However, the accuracy of such volume measurements is limited [19, 20], and respiratory inductive plethysmography provides no information about air leakages. By contrast, precise tidal volume (V T) and leak measurements require a flow sensor in the breathing circuit [21]. Hückstädt et al. were the first to directly measure leak flow and V T during nasal CPAP in neonates [22] using a custom-made differential pneumotachography [23, 24]. More recently, commercial devices for V T and leak measurement during CPAP have become available [25]. However, these have never been tested in the clinical setting, and unlike in adults [9, 10, 14, 15], little is known about the incidence, magnitude and physiological effects of leaks during nasal CPAP therapy in neonates [22, 26]. Therefore, this prospective clinical study aimed to assess the feasibility of V T and leak measurements during nasal CPAP in neonates using a commercial ventilatory device.
Methods
A prospective study was performed at the tertiary neonatal unit at Charité University Hospital CCM from March to September 2008. All inborn neonates on nasopharyngeal CPAP (but not those on binasal CPAP or those with severe malformations of the upper airways) were eligible if they had been receiving CPAP therapy for at least 24 h prior to measurements. The study protocol was approved by the Clinical Ethics Committee of the Charité University Medicine Berlin (EA1/223/07). Informed consent was obtained from all parents before newborns were included in the study.
Measurement equipment
Tidal volume and leak measurements during CPAP were performed using the Leoni ventilator (Heinen & Löwenstein, Germany), which has recently been validated in a bench test [25]. In short, the Leoni is a ventilator designed for mechanical ventilation and CPAP in neonates, infants and children up to 30 kg. An anemometric flow sensor (R = 7.8 Pa s l−1, dead space 1 ml) located between the Y-piece and the CPAP interface allows airflow measurements during nasopharyngeal or face mask CPAP. Breaths are recognized automatically by analysis of the flow signal, which is integrated breath-by-breath to determine measured inspiratory volume (V in), measured expiratory volume (V ex), respiratory rate (RRvent) and minute volume (V′ E). The displayed volume is V ex, and the leak is calculated by:
The ventilator’s measuring range is limited to leaks <90%. All ventilatory parameters displayed by the Leoni (leak, V ex, RRvent, V′E) are mean values calculated over the previous 60 s. As shown recently [21], leak flow can be calculated as follows:
Presuming leak resistance is constant and much higher than the resistance of the CPAP system, and inspiratory time (T in) equals expiratory time (T ex), the leakage-corrected tidal volume (V Tcorr) can be calculated as follows [21]:
The ventilator and a non-heater wire ventilatory circuit were connected with the patient in the usual way, using nasopharyngeal tubes with internal diameters of 2.5–3.5 mm (Vygon, Ecouen, France) as CPAP interface. The breathing gas was humidified and heated by an MR850 (Fisher & Paykel, Auckland, New Zealand). Heart rate (HR), saturation of peripheral oxygen (SpO2) and respiratory rate (RRmon) were monitored by an M1094B (Hewlett Packard, Delaware, USA). A K510 digital stopwatch (Kienzle, Hamburg, Germany) was used for timing.
Protocol
Before measurements were taken, all infants received nasopharyngeal CPAP via Babylog 8000 (Dräger Medical, Germany). Calibrations and device checks of the Leoni and the humidifier were performed as described in the user manuals. A warm-up period of at least 15 min was allowed using intermittent mandatory ventilation and a test lung until the recommended airway temperature of 37°C had been reached. The Babylog was disconnected and the Leonie was connected using identical CPAP settings, as previously adjusted by the clinician according to clinical considerations. Median (range) parameters encountered were CPAP = 5 (4–7) cmH2O, flow = 6 (5–8) l min−1 and FiO2 = 0.21 (0.21–0.47).
Leak and V T measurements were performed with and without occlusion of the contralateral nostril in a randomized cross-over trial using a computer-generated randomization list once the equipment had been set up. For nostril occlusion, an Ohropax Soft earplug (Ohropax GmbH, Wehrheim, Germany) made of hypoallergenic, near-airtight polyurethane plastic foam served as basic material and was cut to size using a disposable scalpel. This nose plug was compressed and inserted into the nostril, where it was allowed to expand. Aside from this intervention, the study did not affect clinical management of the patient. One investigator (H.F.) carried out all measurements.
Vital signs (HR, RRmon, SpO2) were documented before connection and before disconnection of the Leoni. During measurements the displayed parameters of the Leoni (leak, V ex, RRvent, V′ E) and of the monitor (RRmon, SpO2) were documented in 1-min intervals over a 10-min period. Furthermore, the newborn’s behavior was documented as “calm” or “restless,” and mouth position as “open” or “closed.” At least 15 min was allowed prior to each 10-min measurement period to attain a steady state.
Statistics
All measured physiological parameters were described by mean and standard deviation (SD) in the text, and by mean and 95% confidence intervals in the figures, unless specified otherwise. Patient characteristics, CPAP parameters and mean leak values were not normally distributed and were described by median and range. Parametric and nonparametric tests for independent and paired samples were used to compare means and medians as indicated. STATGRAPHICS software (version 5.0, Manugistics, Rockville, MA) was used for statistical analysis. A level of statistical significance of p < 0.05 was accepted.
Results
Patients
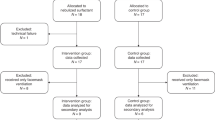
During the 6-month period, 48 patients were eligible for the study. Sixteen patients were excluded because parents did not consent (n = 8), the investigator was not on call (n = 5) or due to technical difficulties (n = 3).
The 32 neonates recruited received CPAP for respiratory distress syndrome (n = 18), wet lung and other transient respiratory insufficiencies of the newborn (n = 9), pneumonia (n = 2), bronchopulmonary dysplasia (n = 1), congenital diaphragmatic hernia (n = 1) and neuromuscular disease (n = 1). Table 1 shows the characteristics of the study population. The majority had received antenatal steroids (n = 27). After delivery, patients were started on primary CPAP therapy (n = 14), were intubated and ventilated (n = 14), or were intubated briefly for surfactant administration (n = 4). Most intubated patients received surfactant (n = 16). One premature infant was only measured at the age of 36 days after the fifth extubation, as previous extubation attempts had been unsuccessful.
Effects on study patients
All neonates tolerated the study well, and no clinical adverse events were detected. There were no statistically significant differences between vital signs before and after the study (HR 149 ± 14 vs. 148 ± 12 min−1, RRmon 52 ± 18 vs. 54 ± 17 min−1, SpO2 92 ± 5 vs. 93 ± 5%). Restlessness was reported in 8.1% of all measurements, but was independent of nostril occlusion. Measured SpO2 was identical with and without nasal occlusion (92 ± 5% in both groups). However, nostril occlusion was associated with a significant decrease in RRmon (49 ± 17 vs. 53 ± 17 min−1, p = 0.008). The sequence of nostril occlusion had no effect on measured ventilatory parameters.
Leak measurements
The measured leak showed considerable inter-subject and intra-subject variability. In 31 infants leaks exceeded the 90% measurement range intermittently (n = 20) or even permanently (n = 11). Analyzable leak values over the whole measuring period were obtained from only one patient. Altogether, 148 of 640 single measurements (23%) were analyzable, as shown in Fig. 1. Twenty-one patients (65.6%) with occluded nostril had analyzable measurements, compared to only four patients (12.5%) with open nostril. Figure 2 shows the influence of nostril occlusion on mean leak values of all analyzable measurements for every study patient; patients without analyzable readings had the leak plotted as >90%. The median of all mean leak values was 77.6% with occluded nostril and >90% with open nostril.
Influence of mouth leak
Mouth opening occurred more frequently with occluded nostril than with open nostril (86.3 vs. 79.7%, p = 0.03), and was associated with a substantial increase of leaks. With open nostril, this relationship could not be studied due to the small number of patients with analyzable measurements. Figure 3 shows the influence of mouth opening on leak measurements with occluded nostril. Mean leak values for open and closed mouth were calculated by analogy with Fig. 2. When the mouth was closed and nostril was occluded, quasi-continuous leak monitoring was possible (98% of all single measurements) with a median (range) leak of 4.7 (0.7–13.5)%. With open mouth and occluded nostril, median leak was 78.9 (0 to ≥90)%. For air leakages <90%, calculated median (range) leak flow according to Eq. 2 was 23 (3–77) ml min−1 with closed mouth and 548 (0–1,394) ml min−1 with open mouth (p < 0.001).
Effect of air leakage on volume measurements
As Table 2 shows, the leak did not affect SpO2, RRmon and RRvent. However, with increasing leak there was a significant decrease in V ex and hence in V′E. If leaks were <20%, the mean V ex related to body weight was 5.8 ± 1.3 ml kg−1. Using the algorithm to correct V ex for air leakages (Eq. 3), there was no statistically significant difference between the aforementioned value and V Tcorr for leaks of <20% (V Tcorr = 5.9 ± 1.2 ml kg−1), or even for leaks of 20–69% (V Tcorr = 5.8 ± 2.2 ml kg−1). However, for leaks of 70–89%, V Tcorr increased considerably (V Tcorr = 9.6 ± 2.2 ml kg−1), as shown in Fig. 4.
Discussion
In this clinical study, reliable V T and leak monitoring during nasopharyngeal CPAP was not possible with the equipment used. Leaks were highly variable and often exceeded the measuring range of the CPAP device, which was limited to <90%. Concerning the analyzable measurements, nostril occlusion and mouth opening significantly affected the measured leak. In the presence of leaks ≥20%, the displayed V ex had to be corrected. The volume error could be corrected up to a leak of 69% by applying a recently developed numerical algorithm.
Leak measurements
In neonates, leaks are commonly displayed as a percentage calculated by the difference between V in and V ex in relation to patient ventilation (Eq. 1). However, different leak definitions are in use, which creates difficulties in comparing CPAP devices [21]. The displayed leak may also be affected by inconstant leaks both during the breathing cycle and from breath to breath, especially if difficulties in breath detection ensue. Moreover, the magnitude of the displayed leak depends on the timing parameters of the breathing cycle [25]. A leak flow display would therefore be more informative [21]. With regard to the Leoni, leak flow can be calculated approximately from the displayed leak and V′E (Eq. 2). In the present clinical study this was often impossible, as the displayed leak almost always exceeded the measuring range when the contralateral nostril was open (Figs. 1, 2). By contrast, when the nostril and mouth were closed, measured leak was negligible and quasi-continuous leak monitoring was possible (Fig. 3). This compares favorably with previous measurements of Hückstädt et al. In their study, 46 of 69 neonates on nasal CPAP had to be excluded due to excessive leak flows, even though the mouth and contralateral nostril were closed by the examiner [22].
Influence of mouth leak
As Fig. 3 shows, mouth leaks were a major reason for the high variability of leaks, and measured leaks increased considerably during mouth opening. Interestingly, mouth opening was observed more frequently with occluded nostril than with open nostril. However, it is unclear whether this difference was due to the neonate actively attempting to regulate the CPAP level or whether air was merely escaping passively through a lax mouth in response to increased exhalation resistance. In both cases, it supports the theory that mouth opening serves functionally as a relief valve by inducing a pressure drop between the CPAP interface and the pharynx [26]. On the other hand, even when the mouth opens, the tongue and soft palate may remain in close apposition and reduce mouth leaks, particularly if nasal CPAP presses the soft palate against the tongue [27]. This may explain why at times only minor leaks were measured, even though the mouth was open (Fig. 3).
Volume measurements
Continuous V T monitoring using a flow sensor between the Y-piece and the endotracheal tube has become standard practice during mechanical ventilation of neonates [28, 29]. As V T measurements are susceptible to leaks around uncuffed endotracheal tubes [28, 30], numerical algorithms have been developed that correct measured V T for leaks of up to 90% [31]. Leaks greater than this are likely during nasal CPAP due to mouth and nose leaks. For the Leoni, a numerical correction has been developed, which mainly depends on V ex, T in, T ex and the displayed leak [21]. As the Leoni neither displays T in nor T ex, this clinical study used a simplified formula for leak compensation (Eq. 3). In the presence of leaks of up to 69%, resulting V Tcorr concurred well with previous measurements in spontaneously breathing newborns (V T = 5.6 ± 1.1 ml kg−1[32]). However, non-physiological values for V Tcorr of >12 ml kg−1 were observed during leaks of 70–89% (Fig. 4), probably due to limitations of the correction formula and difficulties in breath detection. Future studies must investigate the extent to which the volume correction used in this paper can be adapted to other devices.
Clinical implications
In neonates, the clinical effects of leaks during nasal CPAP therapy have not been investigated previously, as no reliable measurement technology was available. This study has shown that V T and leak monitoring was generally possible after nostril occlusion. Nostril occlusion was well tolerated, almost eliminated nose leaks and led to a statistically significant reduction in RRmon. A comparable intervention has been used in adults, where chin straps have successfully reduced mouth leaks and leak-related side effects that impair acceptance of CPAP treatment [10, 14, 33]. In neonates, leak reduction augments CPAP transmission to the pharynx [26] and thus increases pulmonary pressure and alveolar recruitment [34]. However, nothing is known about the effect of leak reduction on pulmonary mechanics, work of breathing and gas exchange in neonates. Leaks may also have a positive effect, in that they flush extrathoracic dead spaces and prevent CO2 retention [11, 15]. Therefore, chin straps and nostril occlusion cannot currently be recommended for clinical practice in neonates, as the long-term effects and safety of these measures have not been studied. Technological advances in measurement equipment are needed to allow continuous V T and leak monitoring without disturbing the newborn. Ideally, such technology would be developed for binasal CPAP, which is increasingly being used in newborns [8, 16, 35]. Dead-space-free measurement equipment suitable for binasal CPAP has been described and may be advantageous [22, 24]. Suitable technical solutions are necessary for measurements during bubble CPAP with its noisy flow signal [36], and for variable flow CPAP using devices based on the Benveniste principle [22, 37], in which the intentional leak impairs measurements.
Limitations of the study
There are several limitations to this study. Firstly, anemometric flow measurements are affected by changes in FiO2 [38]. Using the present equipment, it has been shown that an FiO2 of >0.4 caused relative volume errors of >5% [25]. However, only three patients received an FiO2 of >0.4, meaning that changes in FiO2 had only a minimal impact on the study results. Secondly, the frequent occurrence of non-analyzable flow signals due to excessive leaks means that mean leak values calculated from all analyzable flow signals of a patient merely represented the displayed leak, while the true leak was underestimated to an unknown extent. Thirdly, V Tcorr as shown in Fig. 4 was interpreted based on the assumption that V T remains constant in the presence of leaks, which may not be the case. Ideally, the numerical volume correction should be tested against a gold standard (e.g., face-out body plethysmography [39, 40]) to assess its accuracy. Finally, as this study only assessed one device, no conclusions can be made regarding the reliability of other neonatal CPAP devices.
Conclusions
This clinical study has shown that V T and leak monitoring was not feasible with the tested equipment during nasopharyngeal CPAP in neonates. While reliable volume and leak measurements were only possible in the presence of leaks of up to 69%, highly variable nose and mouth leaks regularly exceeded the measuring range of <90%. Measured leaks corresponded to leak flows of up to 1.4 l min−1, but leak flows may have been much higher for leaks ≥90%. Advances in measurement technology are crucial before investigating in more detail the technical and clinical effects of such high leak flows on CPAP therapy in neonates.
Abbreviations
- CPAP:
-
Continuous positive airway pressure
- FiO2 :
-
Fraction of inspired oxygen
- HR:
-
Heart rate
- RRmon :
-
Respiratory rate measured by monitor
- RRvent :
-
Respiratory rate measured by ventilator
- SpO2 :
-
Saturation of peripheral oxygen
- T ex :
-
Expiratory time
- T in :
-
Inspiratory time
- V′E:
-
Minute volume
- V ex :
-
Measured expiratory volume
- V in :
-
Measured inspiratory volume
- V T :
-
Tidal volume
- V Tcorr :
-
Corrected tidal volume
References
Gau GS, Ryder TA, Mobberley MA (1987) Iatrogenic epithelial change caused by endotracheal intubation of neonates. Early Hum Dev 15:221–229
Davis PG, Henderson-Smart DJ (2003) Nasal continuous positive airways pressure immediately after extubation for preventing morbidity in preterm infants. Cochrane Database Syst Rev CD000143
O’Donnell CP, Kamlin CO, Davis PG, Morley CJ (2006) Endotracheal intubation attempts during neonatal resuscitation: success rates, duration, and adverse effects. Pediatrics 117:e16–e21
Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB (2008) Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med 358:700–708
Hascoet JM, Espagne S, Hamon I (2008) CPAP and the preterm infant: lessons from the COIN trial and other studies. Early Hum Dev 84:791–793
Pelosi P, Rocco PR (2008) Effects of mechanical ventilation on the extracellular matrix. Intensive Care Med 34:631–639
De Paoli AG, Morley CJ, Davis PG, Lau R, Hingeley E (2002) In vitro comparison of nasal continuous positive airway pressure devices for neonates. Arch Dis Child Fetal Neonatal Ed 87:F42–F45
De Paoli A, Davis P, Faber B, Morley C (2008) Devices and pressure sources for administration of nasal continuous positive airway pressure (NCPAP) in preterm neonates. Cochrane Database Syst Rev CD002977
Richards GN, Cistulli PA, Ungar RG, Berthon-Jones M, Sullivan CE (1996) Mouth leak with nasal continuous positive airway pressure increases nasal airway resistance. Am J Respir Crit Care Med 154:182–186
Bachour A, Maasilta P (2004) Mouth breathing compromises adherence to nasal continuous positive airway pressure therapy. Chest 126:1248–1254
Kakkar RK, Berry RB (2007) Positive airway pressure treatment for obstructive sleep apnea. Chest 132:1057–1072
Lellouche F, Maggiore SM, Lyazidi A, Deye N, Taille S, Brochard L (2009) Water content of delivered gases during non-invasive ventilation in healthy subjects. Intensive Care Med 35:987–995
Sherman TI, Blackson T, Touch SM, Greenspan JS, Shaffer TH (2003) Physiologic effects of CPAP: application and monitoring. Neonatal Netw 22:7–16
Rabec CA, Reybet-Degat O, Bonniaud P, Fanton A, Camus P (2004) Leak monitoring in noninvasive ventilation. Arch Bronconeumol 40:508–517
Ruhle KH, Domanski U, Franke KJ, Nilius G (2007) Studies of leakage measurements of automatic CPAP-devices. Pneumologie 61:213–218
Roehr CC, Schmalisch G, Khakban A, Proquitte H, Wauer RR (2007) Use of continuous positive airway pressure (CPAP) in neonatal units—a survey of current preferences and practice in Germany. Eur J Med Res 12:139–144
Elgellab A, Riou Y, Abbazine A, Truffert P, Matran R, Lequien P, Storme L (2001) Effects of nasal continuous positive airway pressure (NCPAP) on breathing pattern in spontaneously breathing premature newborn infants. Intensive Care Med 27:1782–1787
Courtney SE, Aghai ZH, Saslow JG, Pyon KH, Habib RH (2003) Changes in lung volume and work of breathing: a comparison of two variable-flow nasal continuous positive airway pressure devices in very low birth weight infants. Pediatr Pulmonol 36:248–252
Werchowski JL, Sanders MH, Costantino JP, Sciurba FC, Rogers RM (1990) Inductance plethysmography measurement of CPAP-induced changes in end-expiratory lung volume. J Appl Physiol 68:1732–1738
Brown K, Aun C, Jackson E, Mackersie A, Hatch D, Stocks J (1998) Validation of respiratory inductive plethysmography using the Qualitative Diagnostic Calibration method in anaesthetized infants. Eur Respir J 12:935–943
Schmalisch G, Fischer H, Roehr CC, Proquitte H (2009) Comparison of different techniques to measure air leaks during CPAP treatment in neonates. Med Eng Phys 31:124–130
Huckstadt T, Foitzik B, Wauer RR, Schmalisch G (2003) Comparison of two different CPAP systems by tidal breathing parameters. Intensive Care Med 29:1134–1140
Ruttimann UE, Galioto FM Jr, Franke JR, Rivera O (1981) Measurement of tracheal airflow in newborns by a differential flow system. Crit Care Med 9:801–804
Foitzik B, Schmidt M, Windstetter D, Wauer RR, Schmalisch G (1998) Leak measurements in spontaneously breathing premature newborns by using the flow-through technique. J Appl Physiol 85:1187–1193
Fischer HS, Roehr CC, Proquitte H, Wauer RR, Schmalisch G (2008) Assessment of volume and leak measurements during CPAP using a neonatal lung model. Physiol Meas 29:95–107
De Paoli AG, Lau R, Davis PG, Morley CJ (2005) Pharyngeal pressure in preterm infants receiving nasal continuous positive airway pressure. Arch Dis Child Fetal Neonatal Ed 90:F79–F81
Ruhle KH, Nilius G (2008) Mouth breathing in obstructive sleep apnea prior to and during nasal continuous positive airway pressure. Respiration 76:40–45
Main E, Castle R, Stocks J, James I, Hatch D (2001) The influence of endotracheal tube leak on the assessment of respiratory function in ventilated children. Intensive Care Med 27:1788–1797
Castle RA, Dunne CJ, Mok Q, Wade AM, Stocks J (2002) Accuracy of displayed values of tidal volume in the pediatric intensive care unit. Crit Care Med 30:2566–2574
Kuo CY, Gerhardt T, Bolivar J, Claure N, Bancalari E (1996) Effect of leak around the endotracheal tube on measurements of pulmonary compliance and resistance during mechanical ventilation: a lung model study. Pediatr Pulmonol 22:35–43
Herber-Jonat S, von Bismarck P, Freitag-Wolf S, Nikischin W (2008) Limitation of measurements of expiratory tidal volume and expiratory compliance under conditions of endotracheal tube leaks. Pediatr Crit Care Med 9:69–75
Schmalisch G, Wilitzki S, Wauer RR (2005) Differences in tidal breathing between infants with chronic lung diseases and healthy controls. BMC Pediatr 5:36
Bachour A, Hurmerinta K, Maasilta P (2004) Mouth closing device (chinstrap) reduces mouth leak during nasal CPAP. Sleep Med 5:261–267
Richardson CP, Jung AL (1978) Effects of continuous positive airway pressure on pulmonary function and blood gases of infants with respiratory distress syndrome. Pediatr Res 12:771–774
Buettiker V, Hug MI, Baenziger O, Meyer C, Frey B (2004) Advantages and disadvantages of different nasal CPAP systems in newborns. Intensive Care Med 30:926–930
Pillow JJ, Travadi JN (2005) Bubble CPAP: is the noise important? An in vitro study. Pediatr Res 57:826–830
Benveniste D, Pedersen JE (1968) A valve substitute with no moving parts, for artificial ventilation in newborn and small infants. Br J Anaesth 40:464–470
Plakk P, Liik P, Kingisepp PH (1998) Hot-wire anemometer for spirography. Med Biol Eng Comput 36:17–21
Milner AD, Saunders RA, Hopkin IE (1977) Effects of continuous distending pressure on lung volumes and lung mechanics in the immediate neonatal period. Biol Neonate 31:111–115
Edberg KE, Sandberg K, Silberberg A, Sjoqvist BA, Ekstrom-Jodal B, Hjalmarson O (1991) A plethysmographic method for assessment of lung function in mechanically ventilated very low birth weight infants. Pediatr Res 30:501–504
Acknowledgments
The authors thank Jenny Metcalf for linguistic revision of the manuscript and Heinen & Löwenstein, Bad Ems, Germany, for the provision of the CPAP device used in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fischer, H.S., Roehr, C.C., Proquitté, H. et al. Is volume and leak monitoring feasible during nasopharyngeal continuous positive airway pressure in neonates?. Intensive Care Med 35, 1934–1941 (2009). https://doi.org/10.1007/s00134-009-1651-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-009-1651-9