Abstract
Objective
To compare iron lung (ILV) versus mask ventilation (NPPV) in the treatment of COPD patients with acute on chronic respiratory failure (ACRF).
Design
Randomised multicentre study.
Setting
Respiratory intermediate intensive care units very skilled in ILV.
Patients and methods
A total of 141 patients met the inclusion criteria and were assigned: 70 to ILV and 71 to NPPV. To establish the failure of the technique employed as first line major and minor criteria for endotracheal intubation (EI) were used. With major criteria EI was promptly established. With at least two minor criteria patients were shifted from one technique to the other.
Results
On admission, PaO2/FiO2, 198 (70) and 187 (64), PaCO2, 90.5 (14.1) and 88.7 (13.5) mmHg, and pH 7.25 (0.04) and 7.25 (0.05), were similar for ILV and NPPV groups. When used as first line, the success of ILV (87%) was significantly greater (P = 0.01) than NPPV (68%), due to the number of patients that met minor criteria for EI; after the shift of the techniques; however, the need of EI and hospital mortality was similar in both groups. The total rate of success using both techniques increased from 77.3 to 87.9% (P = 0.028).
Conclusions
The sequential use of NPPV and ILV avoided EI in a large percentage of COPD patients with ACRF; ILV was more effective than NPPV on the basis of minor criteria for EI but after the crossover the need of EI on the basis of major criteria and mortality was similar in both groups of patients.
Similar content being viewed by others
Introduction
Controlled randomised trials carried out in settings with different level of care [1–4] have shown that non-invasive positive pressure ventilation (NPPV) is more effective than medical therapy in preventing endotracheal intubation (EI) [1–3], and in-mortality rate [1–4] in COPD patients with acute on chronic respiratory failure (ACRF). It has been reported that iron lung ventilation (ILV) can be used successfully in COPD patients with ACRF [5–8]. Moreover, an observational study [9] suggests that the use of both modalities of non-invasive ventilation can avoid EI in a high percentage of patients needing ventilatory support for ACRF. Randomised control studies comparing the efficacy of the two non-invasive ventilatory techniques in COPD patients with ACRF are lacking. In this multicentre prospective randomised trials, we compared COPD patients with ACRF and moderate to severe respiratory acidosis treated with ILV and NPPV in four respiratory intermediate intensive care units (RICU) in order to evaluate: efficacy of each ventilatory technique when used as first line of treatment, and overall efficacy of the sequential use of both techniques in preventing EI in case of failure of the first technique.
Patients and methods
Study design and patients
This prospective randomised controlled study was performed in patients with COPD in ACRF and moderate to severe respiratory acidosis admitted to the RICUs of Ospedale Careggi Firenze, Ospedale Silvestrini Perugia, Ospedale Rasori Parma and Ospedale Forlanini Roma, from January 1999 to December 2003. The protocol was approved by the local ethics committee; all patients gave informed consensus to participate in the study. The patients with COPD and ACRF presenting on admission PaCO2 > 70 mmHg and pH < 7.30 while breathing room air or oxygen at controlled low flow were considered eligible for the study. The patients were included in the study and randomly assigned to receive ILV or NPPV if arterial blood gases after 1 h of pharmacological and oxygen therapy showed no improvement in respiratory acidosis. The diagnosis of COPD was based on clinical history, physical examination, chest film and supported by spirometric evaluation when available (FEV1 less than 70% predicted and FEV1/VC lower than 70% after bronchodilators, in a clinically stable condition). The Zubrod performance scale score [10] was used for evaluation of the clinical status before admission. The level of severity and of consciousness on admission were evaluated by APACHE II score [11] and Glasgow Coma Scale [12].
Patients with the following criteria were not included in the study: (1) need for immediate EI, (2) a tracheostomy or EI performed before the admission, (3) administration of sedative agents as cause of ACRF, (4) neurological disorders unrelated to hypercapnia or hypoxaemia, (5) cardiogenic pulmonary oedema, (6) restrictive disorders (kyphoscoliosis, neuromuscular disorders, fibrothorax) or asthma in combination with COPD, (7) sleep apnea syndrome and severe obesity (BMI > 35), (8) post-operative conditions or trauma, (9) adult respiratory distress syndrome, (10) acute renal failure, (11) causes of decompensation requiring specific treatment (peritonitis, septic shock, acute myocardial infarction), (12) facial deformity.
The randomization schedule had a blocked design for each centre and was generated by an independent statistician who used random numbers. The patient assignment was made by the use of opaque, sealed envelopes.
Modalities of treatment
Pharmacological treatment
All patients underwent the same pharmacological treatment: inhaled salbutamol (5 mg every 4 h), inhaled ipratroprium or oxytroprium bromide (500 mcg every 6 h), intravenous steroids (prednisolone 40 mg every day for a minimum of 5 day), antibiotics and subcutaneous heparin. Diuretics, correction of electrolyte unbalance, cardiokinetic agents, adequate nutritional support and intravenous aminophylline, were used when necessary. Oxygen was administered, by Venturi mask to obtain an arterial oxygen saturation of 90–94%.
Non-invasive mechanical ventilation
All the centres used the same apparatus to deliver ILV (Iron Lung, Mod.CZ800 and Mod.C 900, and Mod. CA1001, Coppa Co, Biella, Italy), whereas NPPV was administered by BiPAP Vision (Respironics, Monroeville, PA) or Helià (Saime, Savigny Le Temple, France).
Iron lung ventilation
The iron lung settings were similar to those previously reported [8]. Briefly, the ventilator was set to deliver peak inspiratory pressures ranging from −30 to −40 cm H2O and peak expiratory pressures from 10 to 15 cm H2O. Inspiratory pressure was set in each individual patient in order to obtain a tidal volume (VT) of 6 mL/kg−1 intermittently recorded at the mouth by means of a Wright’s ventilograph. In patients with spontaneous respiratory frequency <10 cycles/min−1, the iron lung frequency and the ratio of inspiratory time to total breathing cycle time (Ti/Ttot) were set at 15 cycles/min−1 and 30%, respectively. In the other patients, the respiratory frequency and the Ti/Ttot ratio were individually set according to their spontaneous respiratory frequency in order to facilitate patient-ventilator synchrony. Oxygen was provided by Venturi mask to increase SaO2 level to 90–94%.
Non-invasive positive pressure ventilation
Non-invasive positive pressure ventilation was delivered through a full-face mask (Mirage, ResMed, North Ride, Australia, or Respironics Inc, Pittsburg, PA, USA), by BiPAP Vision or Helià ventilators set in spontaneous timed and pressure support mode, respectively. Pressure assist-control mode was used in case of patient-ventilator asynchrony due to air leaks [13]. Positive end-expiratory pressure (PEEP) was set at 4–5 cm H2O, and the level of pressure support (usually ranging from 12 to 20 cm H2O) was titrated to obtain a tidal volume (VT) of 6 mL/kg−1. Expired tidal volume was monitored in the first 4 h of treatment by connecting a pneumotacograph on the circuit between mask and plateau valve in patients ventilated by Vision, and between mask and expiratory valve in those ventilated by Helià. The FiO2 was adjusted to maintain a SaO2 of 90–94%.
Monitoring and criteria to stop ILV and NPPV
During ILV and NPPV electrocardiographic activity, systemic blood pressure and respiratory rate were monitored in all patients. Respiratory rate was continuously monitored by using ECG electrodes (Impedance Pneumograph) and checked by visual inspection of the attending physicians every 15 min for the first 4 h of the ventilatory treatment. Arterial blood samples were also taken at regular intervals during the ventilatory session; a 60-min sampling frequency was employed during the first cycle of ventilation; thereafter, this frequency was reduced to at least one blood sample per ventilatory session. Ventilator settings were adjusted on the basis of continuous oximetry and measurements of arterial blood gases. Non-invasive ventilation was carried out continuously until a SaO2 > 90% and a pH > 7.35 were obtained with a respiratory frequency reduced from the baseline values and lower than 30 breaths/min. Patients were carefully monitored and ventilatory treatment was provided intermittently for at least 6 h per day. Non-invasive ventilation was stopped when patients maintained stable arterial blood gases (PaO2 > 60 mmHg with FiO2 < 40% or with oxygen flow of 3L/min by nasal prongs and pH > 7.37) and a respiratory frequency <25 cycles/min for at least 24 h from the last session of mechanical ventilation.
End-points of the study
The primary end point of the study was to evaluate the success of ILV or NPPV used as first line of treatment. The secondary endpoints were to evaluate: (1) the rescue power in preventing EI of ILV and NPPV used as second line of treatment in case of failure of the first technique, (2) the duration of mechanical ventilation, (3) the length of RICU and Hospital Stay, and (4) the complications for each ventilatory techniques.
According to Brochard and co-workers [1] failure of the initial ventilatory treatment was defined as presence of criteria for EI. EI was promptly performed in the presence of one major criteria. In the presence of two minor criteria after at least 4 h from the start of mechanical ventilation the patient was moved from the initial ventilatory technique to the other.
Major criteria for EI
These include: respiratory arrest, cardiac arrest, bradypnea (respiratory rate < 8 cpm), psychomotor agitation making nursing care impossible and requiring deep sedation, hemodynamic instability (systolic pressure < 70 mmHg).
Minor criteria for EI
Respiratory rate above 35 breaths/min and above the value on admission, arterial pH below 7.30 and below the value on admission, PaO2 below 45 mmHg despite oxygen therapy, decrease of the Glasgow coma score from the value on admission, intolerance to the ventilatory technique. Intolerance was defined as the absolute opposition of the patient to continue the ventilatory treatment in spite of the repeated reassurance of the nurses and physicians.
EI was not performed when patients or relatives (when patient was unconscious) refused this procedure.
Criteria for discharging from RICU
Ability to breath spontaneously for 48 h after disconnection from the mechanical ventilators, hemodynamic stability and no need of artificial nutrition.
Statistical analysis
The study aimed to recruit 140 patients. This sample size gave the study 80% power of detecting a clinically significant difference in the proportion of patients experiencing treatment failure at the 5% level of significance, on the assumption that 30% of the NPPV Group would fulfil the criteria for treatment failure and that a reduction to 10% in the ILV group would be clinically relevant.
Results are given as means (SD) for normally distributed data and as medians with range for non-normally distributed variables. All tests and P values are two-tailed and were analysed on an intention-to-treat basis. The group means were compared by t tests and medians by the Mann–Whitney U test. Analysis of variance for repeated measurements was used to compare arterial blood gases values and pH at baseline and at 2 and 4 h from the start of mechanical ventilation. Nominal variables were compared by using the Pearson χ 2-test. A P value of ≤ 0.05 was considered statistically significant. Analyses were done by SPSS (version 7).
Results
Patients included in the study
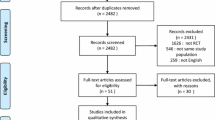
During the period of the study, 2,770 patients were admitted to the four RICUs. Out of a total of 1,297 patients with COPD in ACRF, 297 met the inclusion criteria and among these 156 refused to participate and 141 were randomised to ILV (70 patients) and to NPPV (71 patients) Fig. 1. In Table 1 is reported the number of patients randomised to both treatment by each participating centre. The characteristic of patients on admission are shown in Table 2. The cause of ACRF was pneumonia in 38 patients (19 both in ILV and NPPV group), acute exacerbation in 101 patients (51 in ILV and 50 in NPPV group), and congestive heart failure without clinical and radiological evidence of pulmonary oedema in two patients treated with NPPV.
Clinical outcomes
The rate of success for ILV and NPPV used as first line of treatment was 87% (61/70) and 68% (48/71), respectively (P = 0.01), this difference was due to the number of patients that met minor criteria for EI (2 in ILV and 19 in NPPV groups) (Fig. 2).
ILV failed in nine patients, seven of these met a major criteria for EI whereas two met two minor criteria for EI and were shifted from ILV to NPPV. Among the seven patients who met major criteria for EI two underwent the procedure (1 survived and 1 died), the other five refused (4 died and 1 survived). Among the two patients shifted from ILV to NPPV one was successfully treated, and the other one even though met major criteria for EI refused it and died. The causes of hospital mortality were: cardiac arrest in three patients, cardiogenic pulmonary oedema in two, massive gastrointestinal bleeding in two, aspiration pneumonia in one, ventilator associated pneumonia in one, and massive pulmonary embolism in one.
NPPV failed in 23 patients, four of these met a major criteria for EI, 19 met two minor criteria for EI and were shifted from NPPV to ILV. Among the four patients who met major criteria for EI, three underwent the procedure (two survived and one died) and one refused and died. Among the 19 patients shifted from NPPV to ILV 14 were successfully treated, 5 met major criteria for EI (one survived and four died). The causes of hospital mortality were: cardiac arrest in four patients, gastrointestinal bleeding in two, cerebral haemorrhage in one.
Among the failures of the first ventilatory treatment EI was avoided in 15/21 (71%) of cases when one of the two techniques was used as rescue of the other. The total rate of success using sequentially the two techniques increased from 77.3 to 87.9% (P = 0.028).
After the shift of the ventilatory techniques the need of EI and hospital mortality was similar in both groups of patients (Table 2).
In the patients as a whole, major criteria for EI were present in 12% (17/141), and hospital mortality was 9.9% (14/141).
The duration of mechanical ventilation and hospital stay were similar for ILV and NPPV groups (Table 2).
Physiological outcomes
Arterial blood gases, pH and respiratory rate at the start and after 2 and 4 h of ILV and NPPV are reported in Fig. 3. There was an improvement of arterial blood gases (P < 0.001), pH (P < 0.001), and respiratory rate (P < 0.001) in both group of patients at 2 and 4 h from the start of ventilatory treatment. Arterial pH at 2 and 4 h was significantly higher (P = 0.02 and P = 0.001, respectively) and PaCO2 at 4 h was significantly lower (P < 0.002) in ILV than NPPV group.
Side effects
The rate of total complications in ILV group was 20 vs. 25% of NPPV group (P = 0.506), Table 3.
Discussion
The present data show that: (1) the sequential use of both non-invasive ventilatory techniques avoids the need of EI in a high percentage of COPD patients with ACRF, (2) when used as first line, ILV was more effective than NPPV on the basis of minor criteria for EI, but after the crossover the need of EI and mortality was similar in both groups of patients.
As all previous randomised controlled trials on non-invasive ventilation, this study was not double blinded and this could have influenced the results. However the present one was carried out in specialised RICUs with great expertise in both ILV and NPPV. The patients included in this study had moderate to severe respiratory acidosis (pH < 7.30), as a consequence the results obtained should not be extrapolated to patients with mild respiratory acidosis. According to Brochard et al. [1] failure of the initial ventilatory treatment was defined as presence of pre-defined criteria for EI. It is possible that not all patients who met two or more minor criteria for EI after 4 h from the start of the first ventilatory treatment would have been intubated if not shifted to the alternative technique. However, it must be considered that: (1) the lack of response after the first 2–4 h of NPPV in patients with acute exacerbations of COPD significantly predict the failure of NPPV [14, 15], (2) the current guidelines [16] and recent reviews [17, 18] suggest that in absence of positive response (subjective tolerance and improvement in gas exchange) to NPPV after 2–4 h from the start of treatment the patients should be intubated unless they have a Do Not Intubate Status. In the present study, we used two different ventilators for delivering NPPV; however, the rate of treatment failure was not affected by the type of ventilator used. Finally, we enroled 11% of patients assessed for eligibility due to strict criteria needed to ensure a good balance between the groups. A recent cohort study has shown that the sequential use of ILV and NPPV can be successfully applied in a large unselected patients population with ACRF [9].
The difference in BMI of the two groups of patients even though statistically different was very slight (11%). There is no evidence to our knowledge that this slight difference could have influenced the results.
The rate of failure of NPPV in acute exacerbation of COPD reported in the two most relevant randomized studies was 15 [3] and 26% [1]. More recently in a randomised [19] and a case–control study [20] in COPD patients with severe respiratory acidosis (mean pH 7.20 and 7.22, respectively) the rate of failure of NPPV was 52 and 35%. Even though the comparison with different studies could be subjected to biases due to different criteria used, the rate of failure for NPPV in our study was 32% and this is in line with the severity of respiratory acidosis in comparison to previous studies [1, 3, 19, 20]. The criteria we adopted to establish treatment failure of the ventilatory treatment used as first line were similar to those reported in randomised trials comparing NPPV with standard medical treatment [1, 3]. The difference between previous trials and the present one was that in the presence of minor criteria for EI patients were shifted from one to the other non-invasive ventilatory technique.
In a previous case–control study [8] we found that ILV and NPPV were equally effective in avoiding EI and death in COPD patients with ACRF. These data differ from those of the present study. Several factors may have contributed: (1) in the previous study patients treated with ILV were more severe than those treated with NPPV and different teams employed the two techniques; (2) the previous study has the known limitations of the retrospective design.
The present study confirms and extends previous results [9], and shows that, using both modalities of non-invasive ventilation, only 11.3% of patients met criteria for EI.
This study was carried out in specialized RICUs [21–23]; as a consequence our results could not be generalized to settings with different level of care or with a team not very skilled in ILV.
Our results confirm that complications were infrequent and not significantly different in patients treated with ILV or NPPV [9].
In conclusion, these data show that the sequential use of both non-invasive ventilatory techniques avoids EI in a large percentage of COPD patients with ACRF; although ILV used as first line treatment appears more effective than NPPV on the basis of minor criteria for EI, after the crossover the need of EI and mortality are similar in both groups.
References
Brochard L, Mancebo J, Wysochi M, Lofaso F, Conti G, Rauss A, Simonneau G, Benito S, Gasparetto A, Lemaire F, Isabey D, Harf A (1995) Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 333:817–822
Kramer N, Meyer TJ, Meharg J, Cece RD, Hill NS (1995) Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med 151:1799–1806
Plant PK, Owen JL, Elliott MW (2000) Early use of non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet 355:1931–1935
Bott J, Carroll MP, Conway JH, Keitley SEJ, Ward EM, Brown AM, Paul EA, Elliott MW, Godfrey RC, Wedzicha RC, Moxham J (1993) Randomised controlled trial of nasal ventilation in acute ventilatory failure due to chronic obstructive airways disease. Lancet 341:1555–1557
Corrado A, De Paola E, Gorini M, Messori A, Bruscoli G, Nutini S, Tozzi D, Ginanni R (1996) Intermittent negative pressure ventilation in the treatment of hypoxic hypercapnic coma in chronic respiratory insufficiency. Thorax 51:1077–1082
Corrado A, Gorini M, Ginanni R, Pelagatti C, Villella G, Buoncristiano U, Guidi F, Pagni E, Peris A, De Paola E (1998) Negative pressure ventilation versus conventional mechanical ventilation in the treatment of acute respiratory failure in COPD patients. Eur Respir J 12:519–525
Todisco T, Baglioni S, Eslami A, Scoscia E, Todisco C, Bruni L, Dottorini M (2004) Treatment of acute exacerbations of chronic respiratory failure. Chest 125:2217–2223
Corrado A, Confalonieri M, Marchese S, Mollica C, Villella G, Gorini M, Della porta R (2002) Iron lung versus mask ventilation in the treatment of acute on chronic respiratory failure in COPD patients: a multicenter study. Chest 121:189–195
Gorini M, Ginanni R, Villella G, Tozzi D, Augustynen A, Corrado A (2004) Non-invasive negative and positive pressure ventilation in the treatment of acute on chronic respiratory failure. Intensive Care Med 30:875–881
Zubrod CG, Scheiderman M, Frei E (1960) Appraisal of methods for the study of chemotherapy of cancer in man: Comparative therapeutic trial of nitrogen mustard and triethylene thiophosphoramide. J Chronic Dis 11:7–33
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Teasdale G (1974) Assessment of coma and impaired consciousness: a practical scale. Lancet I:81–83
Calderini E, Confalonieri M, Puccio PG, Francavilla N, Stella L, Gregoretti C (1999) Patient-ventilator asynchrony during noninvasive ventilation: the role of expiratory trigger. Intensive Care Med 25:662–667
Plant PK, Owen JL, Elliott MW (2001) Non invasive ventilation in acute exacerbations of chronic obstructive pulmonary disease: long term survival and predictors of in-hospital outcome. Thorax 56:708–712
Confalonieri M, Garuti G, Cattaruzza S, Osborn JF, Antonelli M, Conti G, Kodric M, Resta O, Marchese S, Gregoretti C, Rossi A (2005) A chart of failure risk for noninvasive ventilation in patients with COPD exacerbation. Eur Respir J 25:348–355
ATS/ERS Task Force Report (2004) Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 23:932–946
Caples SM, Gay PC (2005) Noninvasive positive pressure ventilation in the intensive care unit: a concise review. Crit Care Med 33:2651–2658
Garpestad E, Brennan J, Hill NS (2007) Noninvasive ventilation for critical care. Chest 132:711–720
Conti G, Antonelli M, Navalesi P, Rocco M, Bufi M, Spadetta G, Meduri GU (2002) Noninvasive vs. conventional mechanical ventilation in patients with chronic obstructive pulmonary disease after failure of medical treatment in the ward: a randomized trial. Intensive Care Med 28:1701–1707
Scala R, Nava S, Conti G, Antonelli M, Naldi M, Archinucci I, Coniglio G, Hill NS (2007) Noninvasive versus conventional ventilation to treat hypercapnic encephalopathy in chronic obstructive pulmonary disease. Intensive Care Med 33:2101–2108
Confalonieri M, Gorini M, Ambrosino N, Mollica C, Corrado A (2001) Respiratory intensive care unit in Italy: a national census and prospective cohort study. Thorax 56:373–378
European Respiratory Task Force on epidemiology of respiratory intermediate care in Europe (2002) Respiratory intermediate care units: a European survey. Eur Respir J 20:1343–1350
Carlucci A, Del Mastio M, Rubini F, Fracchia C, Nava S (2003) Changes in the practice on non-invasive ventilation in treating COPD patients over 8 years. Intensive Care Med 29:419–425
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Corrado, A., Gorini, M., Melej, R. et al. Iron lung versus mask ventilation in acute exacerbation of COPD: a randomised crossover study. Intensive Care Med 35, 648–655 (2009). https://doi.org/10.1007/s00134-008-1352-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-008-1352-9