Abstract
Objective
Assessing limits of agreement with helium dilution and repeatability of a new system (lung funcution, LUFU) that measures end-expiratory lung volume (EELV) in mechanically ventilated patients using the O2 washin (EELVWin) and washout (EELVWout) technique. LUFU consists of an Evita 4 ventilator, a side-stream oxygen analyzer, and a dedicated PC software.
Design and setting
Prospective human study in a general ICU of a University hospital.
Patients
Thirty-six mechanically ventilated patients.
Interventions
We obtained 36 couples of both EELVWin and EELVWout measurements in each patient (5 with healthy lungs, 9 with ALI, 22 with ARDS). Measurements were obtained with patients ventilated either by assisted (ASB, 16 measurements) or controlled (CMV, 20 measurements) ventilation. In 19 of 20 cases in CMV, we obtained helium dilution measurements (EELVHe).
Measurements and results
Bias for agreement with EELVHe was −16 ± 156 and 8 ± 161 ml, respectively, for EELVWin and EELVWout. Bias for agreement between EELVWin and EELVWout was 28 ± 78 and 23 ± 168 ml, respectively, for CMV and ASB. During CMV bias for repeatability were 8 ± 92 and 23 ± 165 ml, respectively, for EELVWin and EELVWout. During ASB bias for repeatability were 32 ± 160 and −15 ± 147 ml, respectively, for EELVWin and EELVWout.
Conclusions
The LUFU method showed good agreement with helium, and good repeatability during partial and controlled mechanical ventilation. The technique is simple and safe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alveolar collapse, pulmonary edema with alveolar flooding, pulmonary inflammation, reduction of thoracic compliance are common causes of decrease in functional residual capacity (FRC). Restoration of FRC by means of positive end-expiratory pressure (PEEP), recruitment maneuvers, or prone positioning, is a pivotal principle of modern ventilatory strategy, especially for patients with acute lung injury and acute respiratory distress syndrome (ALI/ARDS) [1]. Theoretically, measurement of FRC (or end-expiratory lung volume during mechanical ventilation with PEEP, EELV) is the ideal method to monitor the effect of ventilatory strategies and the course of a lung disease in terms of alveolar recruitment/derecruitment. However, the real relevance of EELV measurement in critically ill patients, even if suggested by several elements, has still to be proven. This is likely due to the lack of a simple measurement system that would increase the diffusion of EELV assessments and help the clinician and the researcher to become more familiar with these data.
Many systems have been proposed to measure EELV in mechanically ventilated patients. Several investigators have used computed tomography (CT) to measure changes in EELV and to quantify alveolar recruitment both in animal [2] and human studies [3–7]. Unfortunately CT scan needs transfer of the patient to the CT suite, has radiological exposure risks, and is relatively expensive. All these factors limit the possibility of obtaining serial measurements of EELV that would allow close monitoring of the effects of the ventilatory strategy and of the evolution of lung injury.
Alternatively, several techniques based on dilution of tracer gases have been adapted to measure EELV in mechanically ventilated patients [8–19]. Techniques based on rebreathing of a tracer inert gas, e.g. helium, in closed circuit require major modifications in the ventilator breathing circuit [8–11], or discontinuation of basal ventilation [12]. Non rebreathing multibreaths techniques, based on analysis of washin/washout of a tracer gas, e.g. sulphur hexafluoride (SF6) [13, 14], helium [15], nitrogen [16–19], or oxygen [20, 21], require fast and sensitive gas analyzers and a precise synchronization between gas concentration and airway flow signals. Unlike nitrogen and oxygen, foreign inert gases such as SF6 and helium are limited by the need of additional gas tank and dedicated dispensing devices to deliver a constant concentration of gas. Though nitrogen has the advantage over oxygen of being inert and not metabolized, nitrogen fast sensors are relatively expensive for clinical application. On the contrary, fast medical sensors are already available for side-stream oxygen concentration measurements. Thus, one of the problems to be solved remains the synchronization between oxygen concentration and gas flow signals. In order to overcome this problem, Weismann et al. have recently developed an automated method for measuring EELV based on oxygen washin and washout, called LUFU, which uses a side-stream fast oxygen analyzer, the flow sensor of the ventilator, and a mathematical correction for synchronization of the oxygen concentration and gas flow signals [22]. Compared with helium dilution and body plethysmography, the method proved clinically acceptable accuracy in healthy [23, 24] and lung diseased [23] not intubated, spontaneously breathing volunteers. Since the system described requires only a fast response oxygen analyzer and a commercial ventilator, the simple instrumentation required might increase the diffusion of EELV measurement in the clinical practice.
Aim of the present study was to assess repeatability of this new monitoring system in mechanically ventilated patients receiving either total or partial ventilatory support. The new technique was compared with a simplified helium dilution technique that we have recently compared to CT [12].
Materials and methods
Study population
The study population consisted of 36 mechanically ventilated patients admitted to the ICU of San Gerardo Hospital from December 2003 to January 2005. The study protocol was approved by the Institutional Ethical Committee and informed consent was obtained according to the Committee recommendations. Exclusion criteria were: presence of air leaks from bronchopleural fistulae, oxygen inspired fraction (FiO2) higher than 0.8, contraindication (according to the attending physician) to perform an FiO2 change of at least 0.2 (required by the LUFU system), hemodynamic instability.
Among the 36 patients, 20 were undergoing controlled mechanical ventilation (4 volume controlled, VC-PPV; 16 pressure controlled, PC-PPV) while the remaining 16 were on partial ventilatory support (2 airway pressure release ventilation, APRV; 10 pressure support ventilation, PSV, 4 continuous positive airway pressure, CPAP).
Throughout the duration of the study, invasive arterial blood pressure, heart rate, and arterial oxygen saturation (SpO2) were continuously monitored.
Study protocol
Once enrolled in the study, the patients were connected to an Evita 4 ventilator (Draeger, Lubeck, Germany) with respiratory parameters unmodified from those previously set by the attending physician. After a period of stabilization of at least 30 min, a stepwise increase in FiO2 triggered the beginning of the first EELV measurement by O2 washin (EELVWin1), which lasted less than 5 min. Within a few minutes from the end of the procedure, the FiO2 was brought back to the baseline value, and a washout measurement was obtained (EELVWout1). In order to investigate repeatability of the technique, after at least 5 min the procedure was repeated and duplicate measurements of both washin (EELVWin2) and washout (EELVWout2) were obtained.
Moreover, in 19 of the patients undergoing controlled mechanical ventilation, after the washin–washout procedure, two consecutive EELV measurements were obtained 10 min apart by helium dilution technique (see below). To be included in the part of the study comparing O2 washin–washout with helium dilution, patients have to be already paralyzed at the time of study. In none of the patients neuromuscular blockade was initiated or reversed just for the purpose of the study. Conversion to controlled or assisted ventilation was a decision of the attending physician according to the clinical evolution of the patient.
Oxygen washin–washout technique
An extensive description of the technique can be found elsewhere [22–24]. Instrumentation setup consisted of a portable PC connected to an Evita4 ventilator for flow and airway pressure recording, and to a sidestream O2 analyzer suctioning at flow of 200 ml/min and using a fast paramagnetic O2 sensor with a response time to a step change of O2 concentration of 200 ms (Pm1111E, Servomex Group, Crowborough, England). A dedicated software allowed: (1) automatic recognition of a minimum 10% increase (O2 washin) or decreased (O2 washout) in FiO2; (2) automatic match of flow and O2 concentration signals by running a model of sampling instrumentation to correct continuously for sampling delay time, gas viscosity, and sensor response time.
Helium dilution technique
During an expiratory pause, a clampable tube, inserted between the ETT and the circuit Y, was clamped and, after a disconnection from the ventilator, it was connected to a bag containing a 1.5 l mixture of gas with a known concentration of helium (13.44%). A tidal volume was delivered 15 times by periodical compressions of the balloon. At the end of the procedure, the helium concentration in the bag was measured, and EELVHe computed using standard formula [12].
Statistics
Data are presented as mean ± SD, unless otherwise specified. Bland and Altman method was used to evaluate either repeatability between duplicate measurements (washin and washout measurements) or agreement in order to compare the three clinically relevant methods.
Repeatability of two duplicate measurements was assessed by plotting the difference between two consecutive measurements of each parameter versus their mean.
Agreement between the methods (washin vs. helium dilution system, washout vs. helium dilution system, and washin vs. washout) was assessed by plotting the difference between two measurements versus their mean, providing estimation of the bias and its limits of agreement.
The EELV measurements obtained in each patient were grouped according to the presence and severity of lung illness in normal, ALI, and ARDS. Differences between the three groups were assessed by means of one-way analysis of variance between independent groups with Bonferroni correction for post hoc analysis. All tests were made at a significance level of P < 0.05.
Results
Main physiologic characteristics and demographic data of the patients are reported in Table 1. Twenty-two patients had ARDS, nine patients had ALI and five patients had normal lungs and were undergoing mechanical ventilation for extrapulmonary causes (Table 1); five patients had history of COPD. Every patient tolerated well the change in FiO2 required by the washin–washout procedures, without developing significant desaturation or hemodynamic instability during the measurement maneuvers. The ARDS group had significantly lower EELV than both the normal and ALI group; the ALI group had significantly lower EELV than the normal group (Table 1).
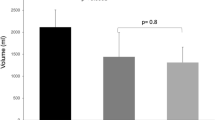
Bias and 95% confidence limits for agreement between washin or washout and helium dilution are reported in Fig 1. The bias between washin–washout method and helium method was not correlated with the level of FiO2, tidal volume, minute ventilation, or respiratory system compliance.
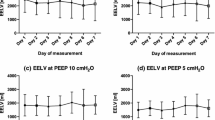
Bland and Altman plots for repeatability of EELVWin, and EELVWout, and for agreement between EELVWin and EELVWout are reported in Fig. 2.
Bland and Altman’s plot of agreement between O2 washin (EELVWin) and O2 washout (EELVWout) (top panel), two repeated measurements of EELVWin (middle panel), and EELVWout (bottom panel). Closed and open circles represent patients undergoing to controlled mechanical ventilation or to partial ventilatory support, respectively. Mean differences (dotted line) and 95% confidence limits (dashed line) are indicated
Biases between EELVWin and EELVWout were 28 ± 78 ml (1.4 ± 3.5%) and 23 ± 165 ml (1.9 ± 9%), respectively, for measurements obtained during controlled and assisted mechanical ventilation.
Biases for repeatability of EELVWin were 8 ± 92 ml (0.1 ± 4.4%) and 32 ± 160 ml (0.8 ± 7%), respectively, for measurements obtained during controlled and assisted mechanical ventilation.
Biases for repeatability of EELVWout were −16 ± 84 ml (−0.7 ± 4.7 %) and −15 ± 147 ml (−1.5 ± 7 %) respectively for measurements obtained during controlled and assisted mechanical ventilation.
Discussion
In the present study, we assessed agreement versus helium dilution and repeatability of the LUFU system, a novel method for the measurement of end-expiratory lung volume in mechanically ventilated patients, based on the O2 washin–washout procedure. Compared to the helium dilution method both O2 washin and O2 washout showed good agreement with an absolute percentage difference from measurements by helium of 8%. The LUFU system showed good repeatability during both controlled and assisted mechanical ventilation.
The first report describing measurement of FRC by an O2 washin procedure dates back to 1982 with the seminal work of Mitchell et al. As for any technique using washin–washout of a tracer gas the most serious source of error is the synchronization between tracer gas concentration and airway flow. Solution to this issue is particularly difficult for O2 washin–washout measurements, since suitable fast oxygen sensors are available only for side-stream measurements. The LUFU method has been recently devised and described by Weismann et al [22] who improved the original Mitchell’s method exploiting the possibility offered by modern gas sensors and computer technology. Comparing LUFU with helium dilution and body plethysmography in non-intubated spontaneously breathing patients, Maisch et al. [23] and Heinze et al. [24] found comparable accuracy and clinically acceptable reproducibility.
This is the first report of the application of LUFU in intubated critically ill patients. We compared LUFU measurements with those obtained with a helium dilution technique adapted for intubated patients that we have recently validate against CT. Since we have validated the helium dilution technique only in patients undergoing controlled mechanically ventilation, comparison between LUFU and helium dilution was limited to paralyzed patients. Both O2 washin and washout measurements showed good agreement compared to helium dilution with 95% confidence intervals for absolute differences within 400 ml. Even better was the agreement between O2 washin and washout measurements. Agreement between O2 washin and O2 washout was comparable to repeatability of single techniques. This is important, since with a single FiO2 change procedure during which FiO2 is changed and then restored we obtain two EELV measurements (one by O2 washin and the other by O2 washout) that could be averaged to decrease measurement errors.
Bias and 95% CI for repeatability of both washin and washout techniques were better during controlled than assisted mechanical ventilation. This may be due to the more variable flow profiles during spontaneous breathing. However, it might also be possible that during spontaneous breathing a true variability of EELV exists [18].
We have studied a population of patients with different degree of pulmonary disease, ranging from healthy lungs to ALI/ARDS, with a wide range of FiO2, PEEP, tidal volume and respiratory rate levels. Values of EELV measured were consistent with the clinical severity of respiratory failure: patients with ARDS had a significantly lower EELV, in spite of significantly higher PEEP, than those with ALI. Other investigators have reported average values of EELV for ALI/ARDS patients ranging between 1,000 and 2,000 ml [3, 12, 17, 25]. Though the average EELV in our study was slightly below 2,000 ml, a comparison with other studies is rather difficult since many factors, not always reported (PEEP level, type of injury, ventilatory mode, ventilatory management previous to the study, tidal volume, size of the patient), greatly affect EELV. Taking in account some of these factors, EELV measured in this study are in our opinion, in line with those measured with different techniques by other investigators in patients with ALI/ARDS [3, 12, 17, 25].
Weismann et al. showed in vitro that at low tidal volumes (less than 300 ml), i.e. with lower respiratory system compliance, the accuracy of LUFU is decreased while the repeatability is unaffected. In our study, the tidal volume ranged from 250 to 620 ml. Differences between LUFU and helium dilution, as well as between repeated LUFU measurements, were not correlated with tidal volume, respiratory rate, minute ventilation, or respiratory system compliance.
Previous works did not show a significant influence of the size of FiO2 change on measurement errors. Theoretically, a 0.1-change would suffice in most patients. However, in certain conditions such as low tidal volume, high EELV, low O2 consumption, the difference between inspiratory and expiratory O2 concentration may be too low, especially for the automatic synchronization of O2 concentration with flow signal. We have used a step change in FiO2 of 0.2. This step is high enough to assure an expiratory to inspiratory difference above the sensitivity of the O2 analyzer, and low enough to be safely applied even in ALI/ARDS patients with high FiO2. Indeed, having performed more than 250 measurements in 36 critically ill patients, we have demonstrated that the technique is safe and easy to use.
LUFU has several advantages; no need to use the dilution of a special gas (and of a mass spectrophotometer for subsequent measurements), ability of performing a measurement without introducing mechanical perturbation in the system nor modification of standard ventilatory circuit, use of pressure and flow sensors integrated in the ventilator. A method with comparable features but based on nitrogen washout–washin has been recently devised by Olegard et al. [19]. They used the flow signal from the ventilator, and the end-tidal and inspiratory O2 and CO2 concentration signals from a standard gas monitor. Nitrogen concentration was calculated as the residual gas from O2 and CO2 concentration. The authors showed good accuracy in a lung model and good repeatability in mechanically ventilated patients. However, their method has not been compared with other methods of EELV measurements, and its repeatability has not been tested during partial ventilatory support.
Conclusions
LUFU system showed good agreement with helium dilution and, most importantly, good repeatability. The method is, safe, simple, and applicable to most ALI/RARDS patients that could benefit from EELV measurements. In our opinion the technique is ready to be integrated in a ventilator. This should give to more investigators the possibility to assess whether the knowledge of EELV may really help to set ventilator parameters, to monitor the effects of the ventilatory strategy, and to follow the evolution of pulmonary illnesses.
References
Ware LB, Matthay MA (2000) The acute respiratory distress syndrome. N Engl J Med 342:1334–1349
Pelosi P, Goldner M, McKibben A, Adams A, Eccher G, Caironi P, Losappio S, Gattinoni L, Marini JJ (2001) Recruitment and derecruitment during acute respiratory failure: an experimental study. Am J Respir Crit Care Med 164:122–130
Malbouisson LM, Muller JC, Constantin JM, Lu Q, Puybasset L, Rouby JJ, CT Scan ARDS Study Group (2001) Computed tomography assessment of positive end-expiratory pressure-induced alveolar recruitment in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 163:1444–1450
Rothen HU, Sporre B, Engberg G, Wegenius G, Hedenstierna G (1993) Re-expantion of atelectasis during general anaesthesia: a computed tomography study. Br J Anaesth 71:788–795
Gattinoni L, Pesenti A, Bombino M, Baglioni S, Rivolta M, Rossi F, Rossi G, Fumagalli R, Marcolin R, Mascheroni D, Torresin A (1988) Relationships between lung computed tomographic density, gas exchange, and PEEP in acute respiratory failure. Anesthesiology 69:824–832
Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri M, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G (2006) Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 354:1775–1786
Hedenstierna G (1993) The recording of FRC: is it of importance and can it be made simple? Intensive Care Med 19:365–366
Weaver LJ, Pierson DJ, Kellie R, Bonner B, Craig KC (1981) A practical procedure for measuring functional residual capacity during mechanical ventilation with or without PEEP. Crit Care Med 9:873–877
Macnaughton PD, Evans TW (1994) Measurement of lung volume and DLco in acute respiratory failure. Am J Respir Crit Care Med 150:770–775
Ibanez J, Raurich JM, Moris SG (1983) Measurement of functional residual capacity during mechanical ventilation. Comparison of a computerized open nitrogen washout method with a closed helium dilution method. Intensive Care Med 9:91–93
Di Marco F, Rota Sperti L, Milan B, Stucchi R, Centanni S, Brochard L, Fumagalli R (2007) Measurement of functional residual capacity by helium dilution during partial support ventilation: in vitro accuracy and in vivo precision of the method. Intensive Care Med 33:2109–2115
Patroniti N, Bellani G, Manfio AM, Maggioni E, Giuffrida A, Foti G, Pesenti A (2004) Lung volume in mechanically ventilated patients: measurement by simplified helium dilution compared to quantitative CT scan. Intensive Care Med 30:282–289
Jonmarker C, Jansson L, Jonson B, Larsson A, Wemer O (1985) Measurement of functional residual capacity by sulfur hexafluoride washout. Anesthesiology 63:89–95
East TD, Wortelboer PJM, Van Ark E, Bloem FH, Peng L, Pace ML, Crapo RO, Drews D, Clemmer TP (1990) Automated sulfur hexafluoride washout functional residual capacity measurement system for any mode of mechanical ventilation as well as spontaneous respiration. Crit Care Med 18:84–91
Heldt GP, Peters RM (1978) A simplified method to determine functional residual capacity during mechanical ventilation. Chest 74:492–496
Fretschner R, Deusch H, Weitnauer A, Brunner JX (1993) A simple method to estimate functional residual capacity in mechanically ventilated patients. Intensive Care Med 19:372–376
Wrigge H, Sydow M, Zinserlmg J, Neumann P, Hinz J, Burchardi H (1998) Determination of functional residual capacity (FRC) by multibreath nitrogen washout in a lung model and in mechanically ventilated patients. Intensive Care Med 24:487–493
Zinserling J, Wrigge H, Varelmann D, Hering R, Putensen C (2003) Measurement of functional residual capacity by nitrogen washout during partial ventilator support. Intensive Care Med 29:720–726
Olegard C, Sondergaard S, Houltz E, Lundin S, Stenqvist O (2005) Estimation of functional residual capacity at the bedside using standard monitoring equipment: a modified nitrogen washout/washin technique requiring a small change of the inspired oxygen fraction. Anesth Analg 101:206–212
Eichler W, Schumacher J, Roth-Isigkeit A, Braun J, Kuppe H, Klotz KF (2002) Automated evaluation of functional residual capacity by oxygen washout. J Clin Monit 17:195–201
Mitchell RR, Wilson RM, Holzapfel L, Benis AM, Sierra D, Osborn JJ (1982) Oxygen washin method for monitoring functional residual capacity. Crit Care Med 10:529–533
Weismann D, Reissmann H, Maisch S, Füllekrug B, Schulte am Esch J (2006) Monitoring of functional residual capacity by an oxygen wahin/washout; technical description and evaluation. J Clin Monit Comput 20:251–260
Maisch S, Boehm SH, Weismann D, Reissmann H, Beckmann M, Fuellekrug B, Meyer A, Schulte am Esch J (2007) Determination of functional residual capacity by oxygen washin-washout: a validation study. Intensive care Med 33:912–916
Heinze H, Schaaf B, Grefer J, Klotz K, Eichler W (2007) The accuracy of the oxygen washout technique for functional residual capacity assessment during spontaneous breathing. Anesth Analg 104:498–604
Neumann P, Zinserling J, Haase C, Sydow M, Burchardi H (1998) Evaluation of respiratory plethysmography in controlled ventilation. Chest 113:443–451
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patroniti, N., Saini, M., Zanella, A. et al. Measurement of end-expiratory lung volume by oxygen washin–washout in controlled and assisted mechanically ventilated patients. Intensive Care Med 34, 2235–2240 (2008). https://doi.org/10.1007/s00134-008-1218-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-008-1218-1