Abstract
Background/purpose
Children receiving extracorporeal membrane oxygenation (ECMO) for respiratory failure can have significant fluid overload and renal insufficiency. Addition of inline continuous venovenous hemofiltration (CVVH) could provide additional benefits in fluid management compared to use of standard medical therapies with ECMO.
Methods
Patients with pediatric respiratory failure receiving ECMO with CVVH were case-matched to similar patients receiving ECMO without CVVH to compare fluid balance, medication use, and clinical outcomes.
Results
Twenty-six of eighty-six patients with pediatric respiratory failure on ECMO (30%) received CVVH for >24 h (median 7.5 days on CVVH). Survival was not significantly different between patients receiving CVVH and those who did not receive CVVH (P = 0.51). For ECMO survivors receiving CVVH, overall fluid balance was less than that in non-CVVH survivors (median 25.1 ml kg−1 day−1; range −40.2 to 71.2 vs. 40.2, 1.1 to 134.9; P = 0.028). Time to desired caloric intake was faster in patients receiving CVVH (1 day, 1–5) than in patients who did not receive CVVH (5 days; 1–11; P < 0.001). Patients receiving CVVH–ECMO also received less furosemide (0.67 vs. 2.11 mg kg−1 day−1; P = 0.009).
Conclusions
Use of CVVH in ECMO was associated with improved fluid balance and caloric intake and less diuretics than in case-matched ECMO controls.
Similar content being viewed by others
Introduction
Children requiring extracorporeal membrane oxygenation (ECMO) for respiratory failure may have significant fluid overload and renal insufficiency before or during an ECMO course [1–3]. Illnesses leading to respiratory failure can require large volumes of fluid resuscitation, and once on ECMO, patients can receive large amounts of blood products. Fluid overload is associated with pulmonary edema, worsening lung injury, and increased incidence of multiple organ failure in critically ill patients [4, 5]. Decreased fluid overload can be associated with improved outcomes in neonates on ECMO [6], adults with ARDS [7–9], or children requiring continuous hemofiltration [10]. While native kidney response is preferred, often urine output is suboptimal due to associated renal ischemia or the effects of medications used in the treatment of critical illness [2–4]. Treating or preventing fluid overload in this setting can require aggressive use of diuretics, which has been suggested to worsen outcomes in critically ill adults with renal failure [11]. Fluid restriction is also often employed in management, decreasing optimal caloric intake, which could be detrimental to improving overall outcomes [12].
The addition of in-line continuous venovenous hemofiltration (CVVH) to the ECMO circuit has been suggested as a potential solution for improved fluid homeostasis in concert with native renal diuresis [13, 14]. The specific placement of inline CVVH delivery pre-ECMO pump or post-pump is controversial. Some CVVH arrangements previously recommended also require a separate delivery pump system in parallel with the ECMO circuit [15]. While offering some theoretical advantages, such systems add cost and complexity to the management of the patient receiving ECMO, which could preclude their use by other centers. At our institution, we have increasingly provided continuous inline hemofiltration to assist in fluid management of pediatric respiratory failure patients on ECMO for either fluid overload or renal insufficiency.
We reviewed our experience by comparing patients receiving ECMO with CVVH to those patients from our own institution, with similar diagnoses and severity of illness, who received ECMO without concomitant CVVH. In this exploratory study, we hypothesized that the addition of CVVH to ECMO improved fluid and nutrition balance, decreased ventilator time, and decreased diuretic use and potassium supplementation compared to patients receiving ECMO without CVVH.
Materials and methods
Data collection and statistical analysis
The computerized ECMO Center database of Children’s Healthcare of Atlanta was queried to determine all non-neonatal patients (more than 1 month old) through age 18 receiving ECMO for respiratory failure from 1992 to 2006. All patients with primary ICD-9 codes with a component of respiratory failure were included. Records were reviewed for demographic variables and for concomitant use of CVVH while on ECMO. Patients receiving CVVH for less than 24 h were excluded (three patients). PRISM III scores at time of ECMO cannulation were also calculated from patient variables as a general comparative indicator of severity of illness [16]. The primary indication for initiation of CVVH was categorized as fluid overload, electrolyte imbalance, or renal failure based on physician report. Renal insufficiency was defined as elevated serum creatinine greater than 1.5 times age-based normal values and urine output less than 1 cc kg−1 h−1.
ECMO fluid balance was calculated as the difference between fluids (ml) in and out over total ECMO course divided by weight (kg) divided by total ECMO days (ml kg−1 day−1). This calculation was also repeated to include only overall fluid balance during days on CVVH. Achievement of desired caloric intake was defined as obtaining greater than 90% of daily-calculated patient caloric needs based on staff nutritionist assessment.
A case-matching process was then used to match patients receiving ECMO with CVVH for greater than 24 h with patients receiving ECMO without CVVH. Patients were initially matched based on one of three major diagnostic groups: pneumonia, acute respiratory distress syndrome, and status asthmaticus. Cases and controls were required to come from within the same basic diagnostic groups. Within the major diagnostic categories, identical primary ICD-9 diagnostic code necessitating ECMO was used to match cases and controls if possible. Patients were then matched for (1) similar patient age (±1 year), and (2) similar PRISM III score at the time of ECMO cannulation (±3). Case–controls were selected independently by two of the investigators (NGH and JDF) and only patient pairs being matched identically by both investigators were included. Using this methodology, all case and control patients utilized for analysis were matched for major diagnostic category, age, and PRISM III score.
Statistical analysis was performed [Statistical Package for Social Sciences (SPSS), version 11.0. Chicago, IL: SPSS, Inc., 2001] and results were reported as median values and ranges. Data was evaluated for normality and determined to be nonparametric for all data groups. For case-matched paired patient data, McNemar’s test was used to determine statistical significance between pairs for categorical variables; Wilcoxon Signed Rank test was used to evaluate continuous variables for statistical significance. Both methods are univariate tests of association. Data in ECMO survivors was unpaired and was evaluated using Mann–Whitney rank sum test to evaluate statistical significance for continuous variables. Statistical significance was determined at a P value ≤ 0.05.
Description of CVVH procedure
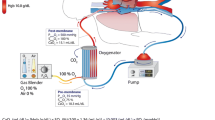
A standardized procedure for CVVH on ECMO is used in our institution as previously described [1]. ECMO priming specialists assemble and prime a hemofiltration circuit and insert it in line with the existing ECMO circuit (Fig. 1). The hemofiltration circuit is constructed using a hemofilter (Renaflow II, Minntech Corp., Minneapolis, MN) or Asahi PAN Hemofilter (Asahi Medical Co., Tokyo, Japan) with CRRT tubing (B. Braun Medical Inc., Bethlehem, PA). The hemofiltration circuit is primed using standard techniques. Ultrafiltrate from the hemofilter is removed using intravenous fluid infusion tubing and an IV pump (I-Med Gemini PC-1, I-Med Corporation, San Diego, CA), which controls the hourly fluid removal rate. Ultrafiltrate is measured by a urine drainage system (Bard CritiCore Collection System, C.R. Bard, Inc., Covington, GA). Actual delivered ECMO blood flow is measured by an ultrasonic flow probe (Transonics Systems Inc., Ithaca, NY) placed on the arterial return tubing of the ECMO circuit. The difference between set ECMO pump flow rate and the delivered pump flow represents the blood flow shunted through the hemofilter.
Schematic diagram of inline hemofiltration apparatus added to ECMO circuit. Blood from membrane oxygenator is drained via three-way stopcock and pigtail tubing (a) to hemofilter. Alternate connection (b) to hemofilter can be made after intial cannulation. Hemofilter circuit blood returns to circuit via tubing (c) and three-way stopcock to venous bladder chimney. Hemofilter ultrafiltrate is drained via automated pump (d) and collected and measured via urometer. Electronic flow probe (f) measures actual postmembrane flow for calculation of hemofilter “runoff”
Results
Eighty-six pediatric respiratory failure patients received ECMO support during the time period studied. Fourteen (16.3%) received VA ECMO and 72 (83.7%) received VV ECMO. Overall survival in patients receiving ECMO from 1992 to 2006 was 76% (65/86). Survival in patients receiving ECMO from 1998 to 2006 was 67%.
Twenty-six patients (30% of respiratory failure ECMO) received CVVH for more than 24 h and were included for further analysis. Documented physician indications noted for CVVH were fluid overload (18), electrolyte imbalance (3), and renal failure (5). Ten patients met defined criteria for renal insufficiency at the time of CVVH initiation. Median age of patients receiving ECMO with CVVH was 24.5 months (range 1 month–17.5 years). Median days on CVVH during the course of an ECMO run were 7.5 (1–30). Median duration of CVVH as a percentage of ECMO course was 88% (range 14–100%). Twenty-two of the twenty-six patients received CVVH for more than 40% of total days on ECMO. Only 10% of patients received CVVH for the entirety of their ECMO run.
Twenty-six patients receiving ECMO without CVVH were identified who met case–control criteria with patients receiving ECMO/CVVH (Table 1). Case–control CVVH/non-CVVH pairs included 15 with a major diagnosis of pneumonia, 10 with ARDS, and 1 with status asthmaticus. Pairs were also adequately matched for age and PRISM III scores as defined in “Materials and methods.” Days on ECMO, use of VV ECMO, and patient survival were not significantly different between non-CVVH and CVVH groups. However, more patients in the ECMO/CVVH group required vasopressors prior to cannulation (P = 0.04).
Overall fluid balance was similar between the two groups when evaluating on all ECMO days. However, fluid balance was significantly lower for patients receiving ECMO/CVVH (median 17 ml kg−1 day−1, range −59.1 to 196) compared to patients receiving ECMO alone (37.8, −11.1 to 135; P = 0.022) when calculated only for days on CVVH (Fig. 2). Overall ECMO fluid balance in CVVH/ECMO survivors was less than that in non-CVVH survivors (median 25.1 ml kg−1 day−1; range −40.2 to 71.2 vs. 40.2, 1.1 to 134.9; P = 0.028) (Fig. 2), as well as for days on CVVH alone (14 vs. 40.2 ml kg−1 day−1; P = 0.005).
Box plot of fluid balance. Values in ml kg−1 day−1 are represented by box with box closest to zero indicating 25th percentile, line within box marking the median, boundary farthest from zero indicating 75th percentile, whisker bars indicating 10th and 90th percentiles, and dots representing outliers. *P = 0.015 for ECMO/CVVH days only versus non-CVVH, **P = 0.05 for ECMO/CVVH survivors versus ECMO/non-CVVH survivors, #P = 0.005 for ECMO/CVVH days only versus ECMO/non-CVVH survivors
Other outcome variables were evaluated for patients receiving ECMO (Table 2). Patients receiving ECMO/CVVH achieved desired caloric intake significantly more rapidly than patients receiving ECMO without CVVH (P < 0.001). Patients receiving CVVH/ECMO (and survivors alone) also received significantly less furosemide than those receiving ECMO alone. Potassium supplementation requirements were not significantly different between groups (Table 2). Total ventilator days post-ECMO also did not differ between patients receiving ECMO with CVVH and patients receiving ECMO without CVVH or survivors alone. No ECMO survivor receiving CVVH developed chronic renal failure or required chronic renal replacement therapy.
Additional analysis was done for the subgroup of patients receiving ECMO/CVVH, who received CVVH for more than 40% of their ECMO duration (n = 15). Similar decreases in fluid balance were seen compared to patients receiving ECMO without CVVH, but these were not different from the total CVVH/ECMO group.
Discussion
Several pediatric series have directly described experience with use of CVVH in ECMO. Sell et al. reported that the addition of hemofiltration in six neonates with acute renal failure on ECMO improved management of oliguric renal failure and weight gain, but only two of the patients survived [13]. A similar report found continuous ultrafiltration effective in neonates on ECMO [2]. In a larger pediatric series [1], 18 of 128 patients with pediatric respiratory failure on ECMO received hemofiltration, with 55% survival. A report of 35 neonatal and pediatric patients receiving hemofiltration for renal insufficiency while on ECMO found that approximately 43% of patients survived, and 14 of the 15 survivors had complete renal recovery [14]. These authors suggested that CVVH, contrary to prior concerns, did not decrease survival or prevent renal recovery compared to historical patients receiving ECMO, who did not receive hemofiltration. A larger recent series of 53 children receiving CVVH on ECMO demonstrated center survival of 34%, compared to 53% of children receiving CVVH alone [17]. However, none of these reports of hemofiltration on ECMO compared patients matched for severity of illness, nor was the actual effect on fluid balance evaluated.
We found in our series that concurrent use of CVVH for assistance in fluid management in patients receiving ECMO was associated with improved fluid balance and caloric intake with less use of diuretics compared to similar patients receiving ECMO who receive standard ECMO without CVVH. While interesting, our findings do not provide adequate evidence to recommend routine use of CVVH in this setting. We did not demonstrate differences in survival or duration of ventilation in patients receiving CVVH. In fact, it is likely that adding CVVH in a nonrandomized fashion reflects a relatively higher severity of illness in children chosen to have CVVH added. While PRISM scores were essentially the same with the case–control pairs, need for inotrope support was significantly greater in patients receiving ECMO/CVVH, likely reflecting more extensive organ insufficiency. Given this likely disparity, the evidence of decreased fluid need in patients receiving ECMO/CVVH could be understated, and outcome benefits could possibly be increased more significantly in a study performed in randomized fashion.
Recent studies have suggested that improved fluid balance could be associated with improved outcomes in critically ill patients. An early study suggested that the mobilization of fluid was associated with better survival in neonates on ECMO for respiratory failure [6]. Weber and Kountzman found that the inability to reach pre-ECMO dry weight was a prognostic factor for nonsurvival in their series [1]. They noted that survivors (mean weight loss 5 ± 2%) demonstrated improved fluid balance on ECMO compared to nonsurvivors (mean weight gain 11 ± 5%). A recent report also found that earlier intervention with hemofiltration was associated with improved survival in critically ill children not on ECMO [10].
The potential benefit of CVVH and improved fluid balance in ECMO could arise from several theoretical advantages. Use of CVVH could enhance removal and absorption of undesirable inflammatory mediators in the patient receiving ECMO. However, such effects were not evaluated in the current study. Increased use of CVVH can also potentially allow for decreased use of diuretics for fluid management. A recent retrospective study found that concomitant use of diuretics in adults with acute renal failure was associated with a twofold increase in risk of mortality [11]. The authors suggested that, while the observations prohibited causal inference, diuretics in this setting were unlikely to be of clinical benefit, and could be harmful either due to direct deleterious effects or by causing delay in the initiation of renal replacement therapy [11]. Use of diuretics could present additional risks for ototoxicity in infants. Infants surviving neonatal intensive care unit stays are more likely to demonstrate sensorineural hearing loss, which has been attributed to exposure to multiple medications, including loop diuretics [18]. A recent Canadian follow-up study of neonates surviving severe respiratory failure found that cumulative dosage and duration of furosemide was independently associated with sensorineural hearing loss (P < 0.001) [19]. In our series, we found a significant decrease in diuretic exposure in patients receiving ECMO on CVVH. Diuretic exposure would likely decrease even further with earlier institution of CVVH in the ECMO course.
Finally, improved fluid balance could allow for more aggressive intervention with nutritional support and hasten achieving optimal caloric intake. A recent meta-analysis found that early enteral nutrition was associated with lower incidence of infections, and reduced hospital length of stay in critically ill adults [12].
However, the improvements in fluid balance, nutrition, and diuretic use seen in our comparison pairs were not associated with improved overall survival or decrease in ventilator days. This lack of survival difference could be due to several factors. Primarily, the study was neither designed nor powered to demonstrate a difference in these outcomes. A much larger patient population would likely be required to demonstrate any potential mortality differences. Additionally, this study was not prospective, and CVVH was not routinely initiated at the time of ECMO cannulation (only 10% of patients). It is possible that routine CVVH use over the entire course of an ECMO run could have been associated with other improved outcome measures.
Addition of CVVH to the circuit via additional hemofiltration or hemodialysis machinery could potentially add to the complexity and cost of an already expensive ECMO course. However, the arrangement we describe requires only the inline addition of a hemofilter and does not require any additional dialysis equipment. In our experience, the extra flow required to correct for shunting to the hemofilter is approximately 10% of overall ECMO flow. This flow can be adequately provided with increasing ECMO pump flow based on flow probe determinations. The use of simple “free flow ultrafiltration” with IV pump assistance has been described for use in extracorporeal circuits and also in an experimental setting in a stand-alone setting [20]. However, close attention is required to assess patient level of hydration, as inaccuracy in pump delivery of replacement fluid volume can occur. Concern for inaccuracy in delivery has been noted with use of IV infusion pumps in stand-alone CRRT. Jenkins et al. reported a magnitude of error up to 12.5% when linear peristaltic pumps were impacted by high pump pressure gradients [21]. Other investigators have demonstrated adequate accuracy in children receiving CRRT with or without ECMO [22]. Because of concerns for potential inaccuracy, some ECMO centers opt to utilize standard continuous renal replacement delivery devices placed in parallel with the ECMO circuit. At our center, we have occasionally utilized the Braun Diapact (B. Braun Medical, Inc., Bethlehem, PA) CRRT device in patients receiving ECMO, who weigh more than 30 kg. Adding a further device does add to complexity, cost, and potential for other machine/user errors. Therefore, further studies are in progress with clinical and engineering investigators to calculate these potential inaccuracies [23] and develop a simpler alternative device to improve accuracy in providing CVVH on ECMO.
The majority of patients receiving ECMO in our series received CVVH for indications of fluid overload (62% of all patients receiving CVVH/ECMO) in the absence of defined renal insufficiency (38% of patients receiving CVVH). In comparison, CVVH was initiated for renal failure (using a definition similar to that in our study) in 51% of patients receiving ECMO in Meyer’s series. This could account in part for the better outcomes (73% survival) in our patients receiving CVVH/ECMO compared to those in previous reviews (0–43% survival [14, 17]), given that poorer ECMO outcomes have been associated with the presence of renal failure [1, 24].
In conclusion, use of a standardized form of inline CVVH for fluid management assistance was associated with improved fluid balance and caloric intake and less furosemide use in pediatric respiratory failure ECMO survivors compared to case-matched ECMO control survivors receiving medical therapy alone. These findings alone, however, do not provide adequate evidence to recommend routine use of CVVH in ECMO. These benefits could also reflect care and fluid management in a unit with experience in the technique and a heightened sense of awareness of fluid balance. A randomized trial of inline CVVH in respiratory failure ECMO compared to standard therapy would be necessary to determine if CVVH use decreases ECMO/ventilator duration or overall mortality compared to ECMO with native renal response alone.
References
Weber TR, Kountzman B (1998) Extracorporeal membrane oxygenation for nonneonatal pulmonary and multiple organ failure. J Pediatr Surg 33:1605–1609
Heiss KF, Pettit B, Hirschl RB, Cilley RE, Chapman R, Bartlett RH (1987) Renal insufficiency and volume overload in neonatal ECMO managed by continuous ultrafiltration. Trans Am Soc Artif Intern Organs 33:557–559
Roy BJ, Cornish JD, Clark RH (1995) Venovenous extracorporeal membrane oxygenation affects renal function. Pediatrics 95:573–578
Rosenberg AL (2003) Fluid management in patients with acute respiratory distress syndrome. Respir Care Clin N Am 9:481–493
Schuller D, Mitchell JP, Calandrino F, Schuster D (1991) Fluid balance during pulmonary edema: is fluid gain a marker or a cause of poor outcome? Chest 100:1068–1075
Kelly RE, Phillips JD, Foglia RP, Bjerke HS, Barcliff LT, Petrus L, Hall TR (1991) Pulmonary edema and fluid mobilization as determinants of the duration of ECMO support. J Pediatr Surg 26:1016–1022
Mitchell JP, Schuller D, Calandrino FS, Schuster D (1992) Improved outcome based on fluid management in critically ill patients requiring pulmonary artery catheterization. Am Rev Respir Dis 145:990–998
Ware LB, Matthay MA (2001) Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 163:1376–1383
National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network (2006) Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354:2564–2575
Foland JA, Fortenberry JD, Warshaw BL, Pettignano R, Merritt RK, Heard ML, Rogers K, Reid C, Tanner AJ, Easley KA (2004) Fluid overload prior to continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med 32:1771–1776
Mehta RL, Pascual MT, Soroko S, Chertow GM, PICARD Study Group (2002) Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA 228:2547–2255
Marik PE, Zaloga GP (2001) Early enteral nutrition in acutely ill patients: a systematic review. Crit Care Med 29:2264–2270
Sell LS, Cullen ML, Whittlesey GC, Lerner GR, Klein MD (1987) Experience with renal failure during extracorporeal membrane oxygenation: Treatment with continuous hemofiltration. J Pediatr Surg 22:600–602
Meyer RJ, Brophy PD, Bunchman TE, Annich GM, Maxvold NJ, Mottes TA, Custer JR (2001) Survival and renal function in pediatric patients following extracorporeal life support with hemofiltration. Pediatr Crit Care Med 2:238–242
Yorgin PD, Kirpekar R, Rhine WS (1992) Where should the hemofiltration circuit be placed in relation to the extracorporeal membrane oxygenation circuit? ASAIO J 38(4):801–803
Marcin JP, Pollack MM (2000) Review of the methodologies and applications of scoring systems in neonatal and pediatric intensive care. Pediatr Crit Care Med 1:20–27
Shaheen IS, Harvey B, Watson AR, Pandya HC, Mayer A, Thomas D (2007) Continuouus venovenous hemofiltration with or without extracorporeal membrane oxygenation in children. Pediatr Crit Care Med 8:362–365
Rais-Bahrami K, Majd M, Veszelovszky E, Short BL (2004) Use of furosemide and hearing loss in neonatal intneisve care survivors. Am J Perinatol 21:329–332
Robertson CMT, Tyebkhan JM, Peliowski A, Etches PC, Cheung P-Y (2006) Ototoxic drugs and sensorineural hearing loss following severe neonatal respiratory failure. Acta Paediatr 95:214–223
Sanchez C, Lopez-Herce J, Garcia E, Moreno de Guerra M, Moral R, Carrillo A (2001) Continuous venovenous renal replacement therapy using a conventional infusion pump. ASAIO J 47:321–324
Jenkins R, Harrison H, Chen B, Arnold D, Funk J (1992) Accuracy of intravenous infusion pumps in continuous renal replacement therapies. ASAIO J 38:808–810
Ricci Z, Morelli S, Vitale V, DiChiarra L, Cruz D, Picardo S (2007) Management of fluid balance in continuous renal replacement therapy: technical evaluation in the pediatric setting. Int J Artif Organs 30:896–901
Dasi LP, Sucosky P, Goldman S, Paden M, Fortenberry J, Yoganathan AP (2008) Development of a novel fluid management system for accurate continuous hemofiltration in extracorporeal membrane oxygenation. J Med Devices (in press)
Weber TR, Connors RH, Tracy TF Jr, Bailey PV, Stephens C, Keenan W (1990) Prognostic determinants in extracorporeal membrane oxygenation for respiratory failure in newborns. Ann Thorac Surg 50:720–723
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoover, N.G., Heard, M., Reid, C. et al. Enhanced fluid management with continuous venovenous hemofiltration in pediatric respiratory failure patients receiving extracorporeal membrane oxygenation support. Intensive Care Med 34, 2241–2247 (2008). https://doi.org/10.1007/s00134-008-1200-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-008-1200-y