Abstract
Objective
Intravenous immunoglobulin therapy has been proposed as an advanced treatment for sepsis. Yet, its benefit remains unclear and the mechanism of action is poorly understood. One key mediator in the development of sepsis is high mobility group box 1 (HMGB1). Therefore, we examined the serum and lung tissue levels of HMGB1 in a rat model of sepsis.
Design and setting
Prospective controlled animal study in a university laboratory.
Materials
Rats received either cecal ligation and puncture-induced sepsis or had additional intravenous immunoglobulin treatment in boluses of 100, 300, or 1,000 mg/kg.
Measurements and results
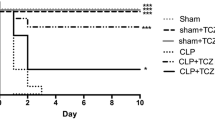
After induction of sepsis and respective treatment conditions, histopathology, wet/dry weight ratios, and signaling molecules were examined in pulmonary tissue. Serum and pulmonary levels of cytokine and HMGB1 were measured. High dose intravenous immunoglobulin (1,000 mg/kg)-treated animals demonstrated significantly improved survival and pulmonary histopathology compared to the control rats. Serum and pulmonary HMGB1 levels were lower over time among intravenous immunoglobulin-treated animals. Furthermore, administration of intravenous immunoglobulin resulted in inhibition of NF-κB activity.
Conclusions
High-dose intravenous immunoglobulin decreased the mortality and pulmonary pathology in a rat model of sepsis. A significant reduction in HMGB1 levels was also observed, which may be mediated by inhibition of inflammation and NF-κB.
Descriptor
23. Acute respiratory distress syndrome (ARDS) and acute lung injury (ALI): experimental models.






Similar content being viewed by others
References
Schlichting D, McCollam JS (2007) Recognizing and managing severe sepsis: a common and deadly threat. South Med J 100:594–600
Andrews P, Azoulay E, Antonelli M, Brochard L, Brun-Buisson C, Dobb G, Fagon JY, Gerlach H, Groeneveld J, Mancebo J, Metnitz P, Nava S, Pugin J, Pinsky M, Radermacher P, Richard C, Tasker R (2006) Year in review in intensive care medicine, 2005. II. Infection and sepsis, ventilator-associated pneumonia, ethics, haematology and haemostasis, ICU organisation and scoring, brain injury. Intensive Care Med 32:380–390
Andrews P, Azoulay E, Antonelli M, Brochard L, Brun-Buisson C, Dobb G, Fagon JY, Gerlach H, Groeneveld J, Mancebo J, Metnitz P, Nava S, Pugin J, Pinsky M, Radermacher P, Richard C, Tasker R, Vallet B (2005) Year in review in intensive care medicine, 2004. I. Respiratory failure, infection, and sepsis. Intensive Care Med 31:28–40
Turgeon AF, Hutton B, Fergusson DA, McIntyre L, Tinmouth AA, Cameron DW, Hébert PC (2007) Meta-analysis: intravenous immunoglobulin in critically ill adult patients with sepsis. Ann Intern Med 146:193–203
Werdan K, Pilz G, Bujdoso O, Fraunberger P, Neeser G, Schmieder RE, Viell B, Marget W, Seewald M, Walger P, Stuttmann R, Speichermann N, Peckelsen C, Kurowski V, Osterhues HH, Verner L, Neumann R, Müller-Werdan U, Score-Based Immunoglobulin Therapy of Sepsis (SBITS) Study Group (2007) Score-based immunoglobulin G therapy of patients with sepsis: the SBITS study. Crit Care Med 35:2693–2701
Abe Y (1996) Therapeutic application of intravenous human natural immunoglobulin preparation. Front Biosci 1:e26–e33
Werdan K (1999) Supplemental immune globulins in sepsis. Clin Chem Lab Med 37:341–349
Disney JE, Johnson KR, Magnuson NS, Sylvester SR, Reeves R (1989) High-mobility group protein HMG-I localizes to G/Q- and C-bands of human and mouse chromosomes. J Cell Biol 109:1975–1982
Bustin M (1999) Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Mol Cell Biol 19:5237–5246
Park JS, Arcaroli J, Yum HK, Yang H, Wang H, Yang KY, Choe KH, Strassheim D, Pitts TM, Tracey KJ, Abraham E (2003) Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am J Physiol Cell Physiol 284:C870–C879
Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH, Suffredini AF (2003) Inflammation-promoting activity of HMGB1 on human micro vascular endothelial cells. Blood 101:2652–2660
Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, Tracey KJ, Yang H (2006) HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock 26:174–179
Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, Ishizaka A, Abraham E (2006) High mobility group box 1 protein (HMGB1) interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol 290:C917–C924
Sunden-Cullberg J, Norrby-Teglund A, Treutiger CJ (2006) The role of high mobility group box-1 protein in severe sepsis. Curr Opin Infect Dis 19:231–236
Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ (1999) HMG-1 as a late mediator of endotoxin lethality in mice. Science 285:248–251
Murakami K, McGuire R, Cox RA, Jodoin JM, Bjertnaes LJ, Katahira J, Traber LD, Schmalstieg FC, Hawkins HK, Herndon DN, Traber DL (2002) Heparin nebulization attenuates acute lung injury in sepsis following smoke inhalation in sheep. Shock 18:236–241
Ward PA (1996) Role of complement, chemokines, and regulatory cytokines in acute lung injury. Ann N Y Acad Sci 796:104–112
Laupland KB, Kirkpatrick AW, Delaney A (2007) Polyclonal intravenous immunoglobulin for the treatment of severe sepsis and septic shock in critically ill adults: a systematic review and meta-analysis. Crit Care Med 35:2686–2692
Hoffmann JN, Fertmann JM, Vollmar B, Laschke MW, Jauch KW, Menger MD (2008) Immunoglobulin M-enriched human intervenes immunoglobulins reduce leukocyte–endothelial cell interactions and attenuate microvascular perfusion failure in normotensive endotoxemia. Shock 29:133–139
Fabrizio K, Groner A, Boes M, Pirofski LA (2007) A human monoclonal immunoglobulin M reduces bacteremia and inflammation in a mouse model of systemic pneumococcal infection. Clin Vaccine Immunol 14:382–390
Ueno H, Matsuda T, Hashimoto S, Amaya F, Kitamura Y, Tanaka M, Kobayashi A, Maruyama I, Yamada S, Hasegawa N, Soejima J, Koh H, Ishizaka A (2004) Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med 170:1310–1316
Hatada T, Wada H, Nobori T, Okabayashi K, Maruyama K, Abe Y, Uemoto S, Yamada S, Maruyama I (2005) Plasma concentrations and importance of High Mobility Group Box protein in the prognosis of organ failure in patients with disseminated intravascular coagulation. Thromb Haemost 94:975–979
Sunden-Cullberg J, Norrby-Teglund A, Rouhiainen A, Rauvala H, Herman G, Tracey KJ, Lee ML, Andersson J, Tokics L, Treutiger CJ (2005) Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med 33:564–573
van Zoelen MA, Laterre PF, van Veen SQ, van Till JW, Wittebole X, Bresser P, Tanck MW, Dugernier T, Ishizaka A, Boermeester MA, van der Poll T (2007) Systemic and local high mobility group box 1 concentrations during severe infection. Crit Care Med 35:2799–2804
Angus DC, Yang L, Kong L, Kellum JA, Delude RL, Tracey KJ, Weissfeld L, GenIMS Investigators (2007) Circulating high-mobility group box 1 (HMGB1) concentrations are elevated in both uncomplicated pneumonia and pneumonia with severe sepsis. Crit Care Med 35:1061–1067
Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, Czura CJ, Wang H, Roth J, Warren HS, Fink MP, Fenton MJ, Andersson U, Tracey KJ (2004) Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA 101:296–301
Yang H, Wang H, Tracey KJ (2001) HMG-1 rediscovered as a cytokine. Shock 15:247–253
Rodriguez A, Rello J, Neira J, Maskin B, Ceraso D, Vasta L, Palizas F (2005) Effects of high-dose of intravenous immunoglobulin and antibiotics on survival for severe sepsis undergoing surgery. Shock 23:298–304
Wang H, Vishnubhakat JM, Bloom O, Zhang M, Ombrellino M, Sama A, Tracey KJ (1999) Proinflammatory cytokines (tumor necrosis factor and interleukin 1) stimulate release of high mobility group protein-1 by pituicytes. Surgery 126:389–392
Liu SF, Malik AB (2006) NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol 290(4):L622–L645
Pritts TA, Moon MR, Wang Q, Hungness ES, Salzman AL, Fischer JE, Hasselgren PO (2000) Activation of NF-kappaB varies in different regions of the gastrointestinal tract during endotoxemia. Shock 14:118–122
Kawai T, Adachi O, Ogawa T, Takeda K, Akira S (1999) Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11:115–122
Adcock IM (1997) Transcription factors as activators of gene transcription: AP-1 and NF-kappa B. Monaldi Arch Chest Dis 52:178–186
Schwartz MD, Moore EE, Moore FA, Shenkar R, Moine P, Haenel JB, Abraham E (1996) Nuclear factor-kappa B is activated in alveolar macrophages from patients with acute respiratory distress syndrome. Crit Care Med 24:1285–1292
Arnalich F, Garcia-Palomero E, Lopez J, Jimenez M, Madero R, Renart J, Vazquez JJ, Montiel C (2000) Predictive value of nuclear factor kappaB activity and plasma cytokine levels in patients with sepsis. Infect Immun 68:1942–1945
Bohrer H, Qiu F, Zimmermann T, Zhang Y, Jllmer T, Mannel D, Bottiger BW, Stern DM, Waldherr R, Saeger HD, Ziegler R, Bierhaus A, Martin E, Nawroth PP (1997) Role of NFkappaB in the mortality of sepsis. J Clin Invest 100:972–985
Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP, Arnold B, Bianchi ME, Manfredi AA, Rovere-Querini P (2005) Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol 174:7506–7515
Acknowledgments
The authors wish to thank Dr. Tomohisa Uchida for giving us helpful advice and for scoring lung injuries.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hagiwara, S., Iwasaka, H., Hasegawa, A. et al. High-dose intravenous immunoglobulin G improves systemic inflammation in a rat model of CLP-induced sepsis. Intensive Care Med 34, 1812–1819 (2008). https://doi.org/10.1007/s00134-008-1161-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-008-1161-1