Abstract
Objective
We examined whether additional helmet flow obtained by a single-circuit and a modified plateau valve applied at the helmet expiratory port (open-circuit ventilators) improves CO2 wash-out by increasing helmet airflow.
Design and setting
Randomized physiological study in a university research laboratory.
Participants
Ten healthy volunteers.
Interventions
Helmet continuous positive airway pressure and pressure support ventilation delivered by an ICU ventilator (closed-circuit ventilator) and two open-circuit ventilators equipped with a plateau valve placed either at the inspiratory or at the helmet expiratory port.
Measurements and results
We measured helmet air leaks, breathing pattern, helmet minute ventilation (\( \dot{\text{V}} \) Eh), minute ventilation washing the helmet (\( \dot{\text{V}} \) Ewh), CO2 wash-out, and ventilator inspiratory assistance. Air leaks were small and similar in all conditions. Breathing pattern was similar among the different ventilators. Inspiratory and end-tidal CO2 were lower, while \( \dot{\text{V}} \) Eh and \( \dot{\text{V}} \) Ewh were higher only using open-circuit ventilators with the plateau valve placed at the helmet expiratory port. This occurred notwithstanding these ventilators delivered a lower inspiratory assistance.
Conclusions
Additional helmet flow provided by open-circuit ventilators can lower helmet CO2 rebreathing. However, inspiratory pressure assistance significantly decreases using open-circuit ventilators, still casting doubts on the choice of the optimal helmet ventilation setup.
Similar content being viewed by others
Introduction
The helmet is a novel interface that may help to improve comfort, reduce local side effects, and increase success rate during continuous positive airway pressure (CPAP) and noninvasive ventilation (NIV) [1]. The efficacy of helmet CPAP and pressure support ventilation (PSV) has been demonstrated in patients with acute respiratory failure [2–6], including patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) [7]. However, there is evidence in the latter patients that helmet ventilation is worse than face mask ventilation in removing carbon dioxide (CO2) [7]. During CPAP, CO2 rebreathing can be minimized by using continuous high flow devices [8, 9]. This depends on the high proportion of fresh gas flowing through the helmet to the expiratory CPAP valve. In contrast, CO2 rebreathing can be substantial both during helmet CPAP [9] and during helmet PSV with intensive care unit (ICU) ventilators (closed-circuit ventilators), even after the manipulation of the pressurization rate and the inspiratory pressure level [10, 11].
To exploit fully the potential benefits offered by the helmet, we tested the hypothesis that CO2 wash-out can be minimized during CPAP and NIV by increasing the helmet airflow using turbine-based, single-circuit ventilators provided with an intentional leak device (open-circuit ventilators) compared to closed circuits previously used [7, 9, 10]. We also tested the hypothesis that the location of the intentional leak device at the helmet expiratory port and not at its more usual position (between the ventilator circuit and the patient-ventilator interface) is crucial to increase the helmet flushing. Finally, since the effectiveness of NIV provided by the helmet is greatly affected by the subject–ventilator synchrony and by the helmet pressurization rate [10, 12], we also assessed the effect of open-circuit ventilation on the above variables.
Materials and methods
Study population
The study included 10 healthy nonobese, nonsmoking volunteers (age 34.4 ± 9.1 years). All subjects were naive to the study's purpose. The local ethics committee approved the study. Written informed consent was obtained from all participants.
Measurements
Airflows were measured using heated pneumotachographs (Fleisch no. 2; Fleisch, Lausanne, Switzerland) connected to differential pressure transducers (Diff-Capp, ±1 cmH2O, Special Instruments, Nordlingen, Germany). Airway opening pressure (Pao) was measured with a differential transducer (Digima-Clic, ±100 cmH2O, Special Instruments). The concentration of CO2 was measured by a side-stream CO2 analyzer (Datex-Ohmeda type F-FM, Instrumentarium, Helsinki, Finland). All signals were collected on a personal computer through a 12-bit analog-to-digital converter (National Instrument DAQCard 700, Austin, TX, USA) at a sampling frequency of 200 Hz (ICU-Lab, KleisTEK Engineering, Bari, Italy).
Experimental setup
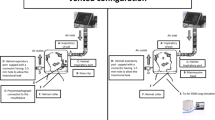
A schematic representation of the experimental setup is shown in Fig. 1. A mouthpiece was connected to a pneumotachograph to measure subject's airflow, with a side port connector for CO2 measurement positioned distally. Measuring equipment dead space was 100 ml. A standard helmet [10], expressly developed for NIV (CaStar “R” StarMed, Italy), was used. Helmet size was chosen to fit volunteer's neck. Helmet airflow and Pao were measured proximally to helmet's inspiratory port. Nonintentional air leaks, i. e., leaks at the subject–helmet interface, were estimated using the ventilator's leak measurement systems. A plateau valve (Respironics, Murrysville, PA, USA) was used with open-circuit ventilators (Fig. 1). This is an exhalation device designed to minimize rebreathing [13].
Schematic representation of the experimental set up. White arrows outside the helmet air flow during inspiration and expiration; arrows inside the helmet flow inside the helmet. A, Pneumotachograph measuring helmet airflow; B, noseclip; C, airway opening pressure; D, plateau valve; E, port tap; F, mouth-piece; G, CO2 sampling catheter; H, CO2 analyzer; I, pneumotachograph measuring subject's airflow. Black arrows: Expanded view of the plateau valve applied at the helmet's inspiratory port (middle panel) and at the helmet's expiratory port (right panel). In this last experimental setup the plateau valve was distally occluded by using a tap, allowing the exhalation device to work properly (white vertical arrow)
NIV and CPAP were delivered by a closed-circuit ventilator (Evita4, Draeger, Lübeck, Germany) and two open-circuit ventilators: the Bipap Vision (Respironics) and the Supportair (Airox, Pau Cedex, France). The closed-circuit ventilator consisted of inspiratory and expiratory lines of the ventilator circuit connected to two dedicated ports placed on right and left sides of the helmet (Fig. 1, left panel). The open-circuit ventilators consisted of a single-circuit connected to the helmet's inspiratory port with the plateau valve randomly placed (a) at the helmet's inspiratory port between the flow transducer and the inspiratory circuit (Airoxinsp and Visioninsp; Fig. 1, middle panel) and (b) at the helmet's expiratory port and distally occluded to ensure its proper functioning (Airoxexp and Visionexp; Fig. 1, right panel). In both conditions exhalation occurred through the plateau valve.
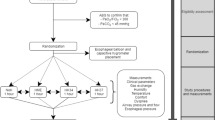
Experimental procedure
A schematic representation of the experimental design is provided in Fig. 2. All subjects were studied while seated, initially breathing room air, wearing a nose clip, through a mouthpiece for about 15 min. Two ventilatory modes were applied with a standard NIV helmet: CPAP 8 cmH2O and PSV 10 cmH2O over PEEP 8 cmH2O using the closed-circuit ventilator and the two open-circuit ventilators. Both the positions of the exhalation port of the open-circuit ventilators were tested for each ventilatory mode. Thus ten conditions were randomly tested in each subject. After completion of the spontaneous breathing period subjects wore the helmet which was secured to the shoulders by armpit braces, and the ten modes of ventilatory assistance were sequentially and randomly applied. Care was taken to minimize air leaks at the patient-helmet interface.
Schematic representation of the experimental design. CPAP, Continuous positive airway pressure; PSV, pressure support ventilation; Airox insp, Airox ventilator equipped with a plateau valve applied at the inspiratory port of the helmet; Vision insp, Vision ventilator equipped with a plateau valve applied at the inspiratory port of the helmet; Airox exp, Airox ventilator equipped with a modified plateau valve applied at the expiratory port of the helmet; Vision exp, Vision ventilator equipped with a modified plateau valve applied at the expiratory port of the helmet
The ventilator inspiratory triggering threshold was set at the most sensitive level that did not induce autotriggering, except in Vision where the threshold depends on the Auto-Trak Sensitivity (Respironics). The pressure rise time (RT) was set at each ventilator's fastest ramp. Expiratory trigger sensitivity was set at inspiratory flow equal to 25% of peak inspiratory flow, except in Vision, where the threshold depends on the Auto-Trak Sensitivity. All experiments were conducted breathing room air with the ventilators' leak compensation function always switched on. All conditions lasted 15 min each. At the end of each period inspiratory flow was increased, and air leaks were created to remove accumulated CO2 in the helmet until helmet PiCO2 reached 0–1 mmHg.
Data analysis
Tidal volume, inspiratory time, expiratory time, and respiratory rate were determined from the subject's flow tracing. Minute ventilation (\( \dot{\text{V}} \) E) was calculated as tidal volume multiplied by respiratory rate. The helmet's minute ventilation (\( \dot{\text{V}} \) Eh) was derived from the helmet flow tracing. The \( \dot{\text{V}} \) E washing the helmet (\( \dot{\text{V}} \) Ewh) was calculated as \( \dot{\text{V}} \) Eh–\( \dot{\text{V}} \) E [11]. PiCO2 was identified as the plateau inspiratory value of the capnogram and the end-tidal PCO2 (PetCO2) as the highest expiratory value. We estimated the amount of inspiratory assistance during PSV by measuring (a) the triggering delay defined as the time elapsed from the onset of the abrupt deflection of flow tracing and the onset of detectable pressurization [14], (b) the pressure RT defined as time required to reach the ventilator's nominal inspiratory pressure, starting from the onset of detectable pressurization [15, 16], and (c) the percentage of inspiratory assistance (%IA) defined as the proportion of inspiratory time spent at nominal PSV. All parameters were determined as the mean of 5-min continuous recording, collected at the end of each experimental condition, once a stable breathing pattern was observed.
Results are expressed as means ± SD. The distribution analysis performed by the Shapiro–Wilk test allowed the use of the two-way analysis of variance for repeated measures and the Bonferroni/Dunn post-hoc tests (StatView software package, Abacus, Berkeley, CA, USA) to compare the different experimental conditions.
Results
All volunteers completed the study protocol. No adverse effects were observed. Nonintentional air leaks were small and similar in all conditions (< 3 l/min). The experimental records from a representative subject are shown in Fig. 3.
Experimental record illustrating end tidal CO2 partial pressure (PetCO 2) and inspiratory CO2 partial pressure (PiCO 2) during both unassisted breathing and helmet noninvasive ventilation delivered by Evita4 (closed-circuit ventilator) and Vision ventilator (open-circuit ventilator). PCO 2, Capnogram; Flow, subject's airflow; Pao, airway opening pressure; CPAP, continuous positive airway pressure; PSV, pressure support ventilation; Vision exp, Vision ventilator equipped with a modified plateau valve applied at the expiratory port of the helmet. Horizontal line, Zero flow. Note the lower increase in PiCO2 and PetCO2 using the Vision ventilator with a distally occluded plateau valve placed at the helmet expiratory port (Visionexp) during both CPAP and PSV conditions
Breathing pattern and CO2 wash-out
A similar breathing pattern was observed during CPAP and PSV delivered by different ventilators (Table 1). \( \dot{\text{V}} \) Eh and \( \dot{\text{V}} \) Ewh were almost always higher when using open-circuit ventilators with the plateau valve placed at the helmet expiratory port, compared to both the closed-circuit ventilator and the open-circuit ventilators with the plateau valve placed at the helmet inspiratory port. \( \dot{\text{V}} \) Eh and \( \dot{\text{V}} \) Ewh were similar only during CPAP when comparing the Visionexp to the Evita4 (Table 1). No difference was observed between the two open-circuit ventilators in any condition. Finally, \( \dot{\text{V}} \) Eh and \( \dot{\text{V}} \) Ewh were lower with Airoxinsp and Visioninsp than with Evita4 only during CPAP. The average values of PiCO2 and PetCO2 are shown in Fig. 4. Similarly to that shown in Fig. 3, PiCO2 and PetCO2 were significantly lower during PSV and CPAP delivered by the Airoxexp and Visionexp than both Evita4 and Airoxinsp and Visioninsp. Conversely, PiCO2 and PetCO2 did not significantly differ between the two different open-circuit ventilators. Finally, they were similar during CPAP delivered by EVITA4 and by Airoxinsp and Visioninsp.
End-tidal CO2 partial pressure (PetCO 2, a) and inspiratory CO2 partial pressure (PiCO 2, b) during both unassisted breathing, and helmet noninvasive ventilation throughout the protocol. SB, spontaneous breathing; CPAP, continuous pressure airway pressure; PSV, pressure support ventilation; Airox insp, Airox ventilator equipped with a plateau valve applied at the inspiratory port of the helmet; Vision insp, Vision ventilator equipped with a plateau valve applied at the inspiratory port of the helmet; Airox exp, Airox ventilator equipped with a modified plateau valve applied at the expiratory port of the helmet; Vision exp, Vision ventilator equipped with a modified plateau valve applied at the expiratory port of the helmet. Results are expressed as means ± SD). # p < 0.01 Evita4 vs. Airoxexp, § p < 0.01 Airoxinsp vs. Airoxexp, * p < 0.01 Evita4 vs. Visionexp, α p < 0.01 Visioninsp vs. Visionexp, analysis of variance for repeated measures with Bonferroni/Dunn post-hoc test
Ventilator inspiratory pressure assistance
Using open-circuit ventilators the inspiratory pressure assistance was significantly less during PSV due to RT increase and % IA decrease than with the Evita4 ventilator, regardless the plateau valve's position (Table 1). Triggering delay was significantly longer only in the Airoxexp vs. Evita4 condition (Table 1).
Discussion
The results of this study confirm our hypothesis that it is feasible to improve CO2 wash-out during helmet CPAP and NIV by increasing the airflow flushing the helmet. To obtain these results we used open-circuit ventilators equipped with a plateau valve positioned at the helmet's expiratory port.
Breathing pattern and CO2 wash-out
Our data confirm that helmet predisposes to CO2 rebreathing [8–10]. Being the helmet similar to a semiclosed system [9], PiCO2 depends primarily on two factors: (a) the amount of CO2 produced by the subject (VCO2) and (b) the fresh gas flow flushing the environment. Accordingly, at a fixed \( \dot{\text{V}} \)CO2, PiCO2 can decrease only by increasing gas flow rates. In particular, only high gas flows (40–60 l/min) were demonstrated effective in maintaining low PiCO2 values inside the helmet [8, 9]. ICU ventilators, not suited to provide high flow CPAP, can generate considerable CO2 rebreathing, limiting/precluding their use with the helmet to deliver CPAP [9]. Moreover, PSV delivered by ICU ventilators has also been shown to generate higher PiCO2 levels when used with helmet than with mask [10, 11].
The present study confirms previous findings obtained during helmet CPAP and extends them to helmet PSV. We provide evidence that is possible to improve CO2 wash-out, thus reducing PiCO2 and PetCO2 during both CPAP and PSV delivered by helmet by inducing a leak at the helmet expiratory port thanks to open-circuit ventilators. This setup allows helmet gas inflow far in excess to the subject's inspiratory flow such that a significant increase in \( \dot{\text{V}} \) Eh and \( \dot{\text{V}} \) Ewh (Table 1) and greater CO2 elimination occur (Figs. 3 and 4). It is noteworthy that a more conventional positioning of the exhalation valve, i. e., between the ventilator circuit and the helmet, precludes the increase in helmet flow and effective CO2 wash-out and hence the clinical use of this particular setup.
Some technical aspects may have influenced our results. First, even nonintentional air leaks allow additional fresh gas flow to flush CO2 from the helmet, facilitating CO2 clearance [9]. Nevertheless during helmet ventilation the presence of nonintentional air leaks can generate a relevant number of autocycled breaths [10], potentially inducing major subject–ventilator dysynchrony, patient discomfort, and even NIV failure. Furthermore, a variable leak makes CO2 wash-out from the helmet unpredictable. Therefore we decided both to increase predictable and to minimize variable air leaks from the helmet by using a plateau valve positioned at its expiratory port. Second, mainstream CO2 sensor technology can affect CO2 readings in the helmet [9]. For this reason we chose to use a side-stream CO2 analyzer, shown to provide reliable CO2 values in helmet studies [8]. Third, although helmet ventilation was found to be higher during CPAP with Evita4 than with Airoxinsp and Visioninsp, PiCO2 and PetCO2 levels were comparable. Among the potential mechanisms that can explain this finding, the location of the plateau valve, i. e., at the helmet inlet, seems crucial. The continuous flow provided at the helmet inlet may act with a mechanism similar to tracheal gas insufflation in enhancing CO2 removal [17].
Ventilator inspiratory assistance
In general at a given level of inspiratory pressure the tighter the coupling between subject's effort and ventilator pressurization the higher is the unloading [17]. In this respect we found that the degree of ventilatory assistance was further reduced using open-circuit compared to closed-circuit ventilators due to high values of RT, reduced %IA, and increased triggering delay (see Table 1). This may worsen the well known pressurization impairment that affects helmet compared to mask ventilation [10] and hence limit the effectiveness of mechanical unloading. Since (a) data on pressurization characteristics of open-circuit are still more controversial and less complete than those of closed-circuit ventilators [18–20], (b) CO2 rebreathing related increase in minute ventilation may also increase the inspiratory muscle effort [10, 12], and (c) in this study direct measurements of respiratory muscle workload were not performed, it is difficult at present provide precise indications on the effectiveness of helmet open-circuit ventilation in unloading inspiratory muscles compared to closed-circuit ventilators (see also below).
Limitations of the study
Our study has several limitations that merit discussion. First, to test the hypothesis that an increase in airflow inside the helmet by inducing an intentional leak (through a modified plateau valve applied at the helmet expiratory port) provides better CO2 wash-out than closed-circuit ventilation we used two single-circuit, turbine-based ventilators and one double-circuit, closed-circuit ICU ventilator. Turbine-based ventilators have been specifically designed to deal with this kind of leaks and are often capable of adequate triggering and ventilation despite their presence. Also many ICU ventilators feature an NIV specific mode dedicated to manage leaks. NIV modes can correct some or all of nonintentional leaks interference but with wide variations between machines in terms of efficiency [21]. For this reason we chose to study the intentional leak condition only using turbine-based ventilators. Second, results may vary between different brands and types of mechanical ventilators due to manufacturing and performance differences. We did not evaluate all currently available ICU ventilators or turbine-based ventilators in our study. However, evaluating a higher number of ventilators would have been impossible due to the excessive number of conditions to be tested.
Third, quite high values of CPAP (8 cmH2O) were applied to volunteers in our study. However, it was previously demonstrated that pressure levels above 5 cmH2O keep the elastic properties of the helmet constant during assisted ventilation [10]. In addition, it has been shown that increasing the PEEP level decreases delay times in helmet NIV [22]. Furthermore, similar levels of PEEP have been used in similar protocols [8, 9]. Thus we aimed to test the helmet's performance under optimal conditions. Fourth, changes in CO2 production can affect both the changes in PetCO2 and the amount of CO2 inside the helmet. We did not measure CO2 production. However, subjects stayed at rest throughout the procedure and their changes in \( \dot{\text{V}} \) E between conditions were of limited extent. Thus it is unlikely that baseline CO2 production, as well as CO2 produced by respiratory muscle contraction, changed significantly throughout the procedure. Finally, it is also unlikely that the protocol itself affected the results because the conditions were applied randomly and accurate helmet CO2 washout was performed between each condition tested.
Clinical implications and conclusions
In the attempt to transfer the present results to the clinical setting it can be argued that PetCO2 and PiCO2 differences obtained adding a leak at the expiratory port of the ventilator are minor and eventually irrelevant. In this respect it must be noted that the present study was performed in normal subjects during unloaded breathing. In response to an increased PiCO2 normal subjects are free to either keep constant [8] or to increase \( \dot{\text{V}} \) E [9]. The former response may occur in the case of end-tidal CO2 values still within the normal range as found in the present and previous studies [8]. By contrast, a PetCO2 already in the upper range of normality [9] may force subjects to increase \( \dot{\text{V}} \) E to keep PetCO2 within normal limits.
Patients' responses may differ in case of respiratory system loading, especially when coupled with preexisting hypercapnia. Previous work in normal subjects showed that loaded breathing raised PetCO2 notwithstanding a twofold increase in \( \dot{\text{V}} \) E during helmet ventilation compared to mask ventilation [10]. Moreover, (a) it was speculated that the observation of a lack of difference in PaCO2 between mask and helmet ventilation in COPD patients with chronic respiratory failure can be due to a significant increase in \( \dot{\text{V}} \) E (and hence in respiratory workload) in the latter condition [12], and (b) helmet ventilation has been demonstrated to be less effective than mask ventilation in removing CO2 in COPD patients with acute respiratory failure [7]. All these data suggest that the harm of rebreathing inside the helmet is proportional to respiratory mechanics worsening, initially leading to increased respiratory workload due to hyperventilation, and ultimately to CO2 retention. Indeed, mechanical constraints can curtail the capability to increase \( \dot{\text{V}} \) E [23, 24], necessary to keep a constant PetCO2 in the face of an increased PiCO2.
The aim of NIV is to unload efficiently the respiratory muscles. Recent studies performed in healthy volunteers [10] and in stable COPD patients [12] show that the helmet is less efficient in unloading the respiratory muscles than the face mask because of the rebreathing-induced \( \dot{\text{V}} \) E increase observed during helmet ventilation [10, 12]. The present study shows how to reduce helmet CO2 rebreathing by positioning the leak of open-circuit ventilators at the helmet expiratory port. From this point of view CPAP provided by the latter device can be suggested as a valid alternative to continuous high flow CPAP systems since both proved to be superior to CPAP delivered by closed-circuit ventilators [9].
The poor patient–ventilator coupling observed during helmet PSV can also impair the ventilator inspiratory muscle unloading [10, 12]. The present study shows that the ventilatory device with the best performance in terms of CO2 wash-out was also the one with the worst respiratory muscle unloading. Theoretically, inspiratory muscles unloading depends on the relative contribution of the two mechanisms discussed above. To our knowledge, there is still uncertainty on what is better in accomplish both the tasks of reducing CO2 retention and unload respiratory muscles. Further studies are warranted to rule out this dilemma.
References
Hill NS (2004) Non-invasive interfaces: should we go to helmets? Crit Care Med 32:2162–2163
Tonnelier JM, Prat G, Nowak E, Goetghebeur D, Renault A, Boles JM, L'Her E (2003) Noninvasive continuous positive airway pressure ventilation using a new helmet interface: a case-control prospective pilot study. Intensive Care Med 29:2077–2080
Principi T, Pantanetti S, Catani F, Elisei D, Gabbanelli V, Pelaia P, Leoni P (2004) Noninvasive continuous positive airway pressure delivered by helmet in hematological malignancy patients with hypoxemic acute respiratory failure. Intensive Care Med 30:147–150
Squadrone V, Choa M, Cerutti E, Schellino MM, Biolino P, Occella P, Belloni G, Vilianis G, Fiore G, Cavallo F, Ranieri VM, Piedmont Intensive Care Units Network (PICUN) (2005) Continuous positive airway pressure for treatment of postoperative hypoxemia: a randomized controlled trial. JAMA 293:589–595
Antonelli M, Conti G, Pelosi P, Gregoretti C, Pennisi MA, Costa R, Severgnini P, Chiaranda M, Proietti R (2002) New treatment of acute hypoxemic respiratory failure: noninvasive pressure support ventilation delivered by helmet: a pilot controlled trial. Crit Care Med 30:602–608
Rocco M, Dell'Utri D, Morelli A, Spadetta G, Conti G, Antonelli M, Pietropaoli P (2004) Noninvasive ventilation by helmet or face mask in immunocompromised patients: case-control study. Chest 126:1508–1515
Antonelli M, Pennisi MA, Pelosi P, Gregoretti C, Squadrone V, Rocco M, Cecchini L, Chiumello D, Severgnini P, Proietti R, Navalesi P, Conti G (2004) Noninvasive positive pressure ventilation using a helmet in patients with acute exacerbation of chronic obstructive pulmonary disease: a feasibility study. Anesthesiology 100:16–24
Patroniti N, Foti G, Manfio A, Coppo A, Bellani G, Pesenti A (2003) Head helmet versus face mask for non-invasive continuous positive airway pressure: a physiological study. Intensive Care Med 29:1680–1687
Taccone P, Hess D, Caironi P, Bigatello LM (2004) Continuous positive airway pressure delivered with a “helmet”: effects on carbon dioxide rebreathing. Crit Care Med 32:2090–2096
Racca F, Appendini L, Gregoretti C, Stra E, Patessio A, Donner CF, Ranieri VM (2005) Effectiveness of mask and helmet interfaces to deliver noninvasive ventilation in a human model of resistive breathing. J Appl Physiol 99:1262–1271
Costa R, Navalesi P, Antonelli M, Cavaliere F, Craba A, Proietti R, Conti G (2005) Physiologic evaluation of different levels of assistance during noninvasive ventilation delivered through a helmet. Chest 128:2984–2990
Navalesi P, Costa R, Ceriana P, Carlucci A, Prinianakis G, Antonelli M, Conti G, Nava S (2007) Non-invasive ventilation in chronic obstructive pulmonary disease patients: helmet versus facial mask. Intensive Care Med 33:74–81
Hill NS, Carlisle C, Kramer NR (2002) Effect of a nonrebreathing exhalation valve on long-term nasal ventilation using a bilevel device. Chest 122:84–91
Giannouli E, Webster K, Roberts D, Younes M (1999) Response of ventilator- dependent patients to different levels of pressure support and proportional assist. Am J Respir Crit Care Med 159:1716–1725
Chiumello D, Pelosi P, Croci M, Bigatello LM, Gattinoni L (2001) The effects of pressurization rate on breathing pattern, work of breathing, gas exchange and patient comfort in pressure support ventilation. Eur Respir J 18:107–114
Diehl JL, Isabey D, Desmarais G, Brochard L, Harf A, Lofaso F (1999) Physiological effects of alveolar, tracheal, and ''standard'' pressure supports. J Appl Physiol 87:428–437
Kirmse M, Fujino Y, Hromi J, Mang H, Hess D, Kacmarek RM (1999) Pressure-release tracheal gas insufflation reduces airway pressures in lung-injured sheep maintaining eucapnia. Am J Respir Crit Care Med 160:1462–1467
Richard JC, Carlucci A, Breton L, Langlais N, Jaber S, Maggiore S, Fougère S, Harf A, Brochard L (2002) Bench testing of pressure support ventilation with three different generations of ventilators. Intensive Care Med 28:1049–1057
Metha S, Hill NS (2001) Noninvasive ventilation: state of the art. Am J Respir Crit Care Med 163:540–577
Battisti A, Tassaux D, Janssens JP, Michotte JB, Jaber S, Jolliet P (2005) Performance characteristics of 10 home mechanical ventilators in Pressure Support mode. A comparative bench study. Chest 127:1784–1792
Vignaux L, Tassaux D, Jolliet P (2007) Performance of noninvasive ventilation modes on ICU ventilators during pressure support: a bench model study. Intensive Care Med 33:1444–1451
Moerer O, Fischer S, Hartelt M, Kuvaki B, Quintel M, Neumann P (2006) Influence of two different interfaces for noninvasive ventilation compared to invasive ventilation on the mechanical properties and performance of a respiratory system: a lung model study. Chest 129:1424–1431
Younes M (1991) Determinants of thoracic excursions during exercise. In: Whipp BJ, Wasserman K (eds) Exercise: pulmonary physiology and pathophysiology. Lung biology in health and disease, vol 52. Dekker, New York, pp 1–65
Wysocki M, Meshaka P, Richard JC, Similowski T (2004) Proportional-assist ventilation compared with pressure-support ventilation during exercise in volunteers with external thoracic restriction. Crit Care Med 32:409–414
Acknowledgements
The authors thank Claudia Filippini and Michele Mele for their technical support and all volunteers for contributions to our research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Racca, F., Appendini, L., Gregoretti, C. et al. Helmet ventilation and carbon dioxide rebreathing: effects of adding a leak at the helmet ports. Intensive Care Med 34, 1461–1468 (2008). https://doi.org/10.1007/s00134-008-1120-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-008-1120-x