Abstract
Objective
Coated medical devices have been shown to reduce catheter-related infections. We coated endotracheal tubes (ETT) with silver sulfadiazine (SSD), and tested them in a clinical study to assess the feasibility, safety, and efficacy of preventing bacterial colonization.
Design
A prospective, randomized clinical trial, phase I–II.
Setting
Academic intensive care unit (ICU).
Participants
Forty-six adult patients expected to need 12–24 h of intubation were randomized into two groups.
Interventions
Patients were randomized to be intubated with a standard non-coated ETT (St-ETT, n = 23; control group), or with a SSD-coated ETT (SSD-ETT, n = 23).
Measurements and results
Coating with SSD prevented bacterial colonization of the ETT (frequency of colonization: SSD-ETT 0/23, St-ETT 8/23; p < 0.01). No organized bacterial biofilm could be identified on the lumen of any ETT; however, SSD was associated with a thinner mucus layer (in the SSD-ETT secretion deposits ranged from 0 to 200 μm; in the St-ETT deposits ranged between 50 and 700 μm). No difference was observed between the two groups in the tracheobronchial brush samples (frequency of colonization: SSD-ETT 0/23, St-ETT 2/23; p = 0.48). No adverse reactions were observed with the implementation of the novel device.
Conclusion
SSD-ETT can be safely used in preventing bacterial colonization and narrowing of the ETT in patients intubated for up to 24 h (mean intubation time 16 h).
Similar content being viewed by others
Introduction
Ventilator-associated pneumonia (VAP) represents one of the most common nosocomial infection in patients intubated and mechanical ventilated [1, 2]. The endotracheal tube (ETT) appears to be an independent risk factor for VAP [3–5]. After a few hours of intubation and mechanical ventilation (MV), polyvinylchloride ETT become colonized by bacteria, which often organize in a thick biofilm, representing a large reservoir of microorganisms that can enter the lungs and cause pneumonia [6–9].
In a previous study in sheep, we coated the internal surface of the ETT with a thin layer of silver sulfadiazine and chlorhexidine in polyurethane, and bacterial growth was reduced in the lower respiratory tract, ETT, and the entire ventilator circuit for 24 h [10].
A public health notice from the US Food and Drugs Administration regarding potential hypersensitivity reactions to chlorhexidine-impregnated medical devices encouraged us to investigate other materials [11]. We constructed several ETT coatings, and we tested efficacy and safety of those tubes in vitro and in animal studies [12]. ETT coated with silver sulfadiazine in polyurethane (SSD-ETT) proved to be effective in preventing bacterial colonization [13].
Therefore, we evaluated, in a randomized clinical investigation, the safety and effectiveness of SSD-ETT in preventing bacterial colonization of the lumen of the ETT in patients requiring mechanical ventilation for up to 24 h.
Materials and methods
Phase I–II randomized clinical trial
A randomized controlled clinical trial was carried out at the Department of Perioperative and Critical Care Medicine of the Ospedale San Gerardo, University of Milano-Bicocca, Monza, Italy and was approved by the institutional review boards of San Gerardo Hospital and the NHLBI. The Italian Ministry of Health also gave its approval.
Endpoints
The primary goal of this clinical study was to establish whether coating ETT with silver sulfadiazine reduces bacterial colonization and biofilm formation within the ETT in patients intubated and mechanically ventilated for 12–24 h.
The secondary endpoint was the reduction of bacterial colonization in the trachea and bronchi.
Safety of the coated ETT
The safety of the coated ETT was strictly monitored, including any difficulty or failure during the intubation period.
In the literature creams based on silver sulfadiazine have been reported to possibly cause transient leukopenia, mainly involving mature neutrophils [14–25]. However, this appears to be a self-limited phenomenon that does not increase the incidence of infectious complications or affect final outcome. Leukopenia was never observed when devices impregnated with silver sulfadiazine were used (intravenous catheters, urinary catheters). Hemolytic anemia in a G6PDH-deficient patient was reported [26], as well as other rare adverse events such as (a) hypersensitivity reactions [27], (b) allergic contact dermatitis [28, 29], (c) erythema multiforme [30], (d) localized and systemic argyria [31–34], and (e) Clostridium difficile-induced toxic megacolon [35]. All these possible side effects were monitored during the hospital stay.
Entry criteria, randomization and sampling
Forty-six patients undergoing cardiac surgery were screened using the following eligibility criteria: (a) age greater than 18 years; (b) expected need of intubation and ventilation support for 12–24 h; (c) intubation with adult-size ETT (7.5 or 8.0 mm ID); (d) informed consent obtained from the patient.
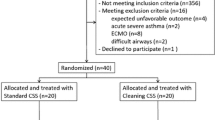
Patients were randomized into two study groups using a computer-generated algorithm. Patients assigned to the control group were intubated with a St-ETT. Patients assigned to the study group were intubated and mechanically ventilated with a SSD-ETT (Fig. 1).
Forty-six cardiac surgery patients were randomized into two study groups using a computer algorithm. Patients assigned to the control group received intubation with a standard, uncoated ETT (St-ETT). Patients assigned to the study group were intubated and mechanically ventilated with an ETT internally coated with silver sulfadiazine (SSD-ETT)
Both groups of patients were kept supine in the operating room, while in the intensive care unit (ICU), the head of the bed was raised 30° if the patients was hemodynamically stable. Patients all received the same perioperative care, including antibiotic prophylaxis according to the American Heart Association guidelines for cardiac bypass surgery patients, intestinal bleeding prophylaxis (40 mg omeprazole twice a day) and continuous measurements of heart rate, blood pressure, central venous pressure, O2 saturation, end-tidal CO2, body temperature, airway pressure and urinary output.
In addition, we recorded the duration of intubation and of ICU stay, incidence of pneumonia, mortality, and adverse events. Microbiology samples were collected from patients at four separate times. First, in the operating room immediately prior to intubation, a sample of oropharyngeal secretions was collected for standard bacterial count. Second, immediately post endotracheal intubation, a sample of secretions was collected by inserting a short catheter into the endotracheal tube and brushing the lumen of the tube for standard bacterial count. Third, immediately prior to extubation, a protected brush sample of tracheobronchial secretions was obtained by fiberoptic bronchoscopy. Finally, after extubation, ETT secretions were collected from the ETT lumen for quantitative bacteriological analysis. A section of the distal ETT was cut (28–29 cm from the ETT connector piece), immersed and fixed in 2.5% glutaraldehyde solution, stored at 4°C and sent for scanning electron microscopy to the Laboratoire de Microscopie Electronique, University of Reims, INSERM ERM 0203, Reims, France. The ETT was also cut from 27–28 cm from the ETT connector piece and immersed in 4% formalin for biofilm fixation, stored at 4°C, and sent for confocal laser microscopy to the Center for Biofilm Engineering, Montana State University, Bozeman, MT, USA.
Pneumonia was defined as new, persistent or progressive infiltrate(s) on chest X-ray(s) consistent with pneumonia, along with at least two of the three following signs: (1) temperature >38.0°C or <35.0°C; (2) leukocyte count ≥ 10,000/mm3 or ≤ 4,500/mm3; or (3) macroscopic appearance of purulent sputum or tracheal secretions. The diagnosis of pneumonia was confirmed by the presence of ≥ 104CFU/ml of bacteria on bronchial alveolar lavage.
ETT coating procedure
The coating procedure was described in detail previously [13]. In summary, we prepared a dispersion of 53 g of silver sulfadiazine and 22.5 g of polyurethane (BioSpan) in 210 ml of N, N-dimethylacetamide. We inserted a standard 8-mm ETT into a hollow transparent acrylic tube to keep the ETT straight. With the plastic tube positioned vertically, we immersed the ETT tip into the dispersion, rapidly aspirated the dispersion up to the level of the connector piece, and then let the ETT drain for 2–4 s. We then placed the transparent plastic tube with the ETT horizontally into a rotating device, through which a stream of air was gently passed to dry the dispersion. After 12 h, the coated ETT was removed and sterilized with ethylene oxide gas. Lumina of the SSD-ETT and St-ETT are shown in Fig. 2.
Confocal scanning laser microscopy. a, b New (never-used) standard commercially available 8-mm-ID ETT: a lumen of a St-ETT; b cross section of a St-ETT. Note the porosity of the polyvinyl chloride. c, d Cross section of new (never-used) ETT coated with silver sulfadiazine dispersion in polyurethane. Note the granular features of the dispersion. Thickness of coating is approximately 40 μm
Microscopy: scanning electron microscopy and confocal laser microscopy
Scanning electron microscopy
The ETT sections in 2.5% glutaraldehyde solution were dehydrated in graded alcohol. We then used 50%–50% hexamethyldisilazane (HMDS; EM Sciences, Fort Washington, PA, USA), and in the end 100% HMDS and air-dried. The samples were sputter-coated (MED 010, Balzers Union) with 15 nm of gold and examined using a Hitachi S-4500 scanning electron microscope equipped with a cold cathode field emission gun at an accelerating voltage of 1.0 kV. All images were recordings using the secondary electron detector. Micrographs were recorded on a PC using PCI quartz cards.
Confocal laser microscopy
The ETT samples were stained with BacLight Live/Dead for 2 h, rinsed with water, then imaged with confocal microscopy using a 20 × 0.4 NA objective. The cross section and the internal coating of the specimen were viewed with a laser scanning confocal microscope.
Statistical analysis
The sample size of 23 subjects per study arm was chosen to assure 90% power with α = 0.05 (two-sided) to detect differences in bacterial colonization of the endotracheal tube. We used the Wilcoxon (Mann–Whitney) rank sum test for group comparisons of continuous variables. Fisher's exact test was used for the analysis of categorical variables. A p value < 0.05 was considered statistically significant. All tests were two-sided. We performed all analyses with the Stata statistical package (Stata Corporation, College Station, TX; release 8.0).
Results
Demographics, clinical results, safety
Forty-six patients met the criteria and were randomized and enrolled in this clinical trial; no patient was subsequently excluded, and none withdrew consent. Twenty-three patients were intubated with a standard ETT (n = 23; St-ETT group, control group), and 23 with a coated SSD-ETT (n = 23; SDD-ETT group, study group) (Fig. 1). The two study groups had similar baseline characteristics, lengths of intubation, ICU stay, and incidences of post-surgery atelectasis and nosocomial pneumonia (Table 1). No adverse events related to the use of silver sulfadiazine were observed during the period of intubation or at any time during the hospital stay (Table 1).
Bacteriology
Prior to anesthesia in the operative room, commensal bacteria colonized all patients' oropharyngeal secretions with no difference between the two groups. Upon intubation, ETT brushing were sterile in all samples of both groups.
At the time of extubation, 8 (35%) out of 23 St-ETT were colonized with a bacterial growth ranging between 0 and 1.0× 107 cfu/ml. None of the SSD-ETT was colonized at extubation (0%; p < 0.01).
In the SSD-ETT group no tracheobronchial brush sample was colonized, while two tracheobronchial brushes of the St-ETT group (two patients) were colonized (p = 0.48) (Table 2). Both patients with colonized tracheobronchial brush also had ETT samples colonized with the same bacterial species (α-hemolytic Streptococcus spp. in one case, Branhamella catarrhalis in the other). Two days after extubation, the patient with Branhamella catarrhalis in the tracheobronchial brush developed hospital-acquired pneumonia; sputum was positive for Branhamella catarrhalis. The pneumonia was treated with antibiotics, and the patient recovered.
The most common aerobic bacteria found in the oral secretions, lumen of the ETT and tracheobronchial brush included: Acinetobacter, Branhamella catarrhalis, Haemophilus spp., Neisseria spp., and α-hemolytic Streptococcus spp.
Microscopy
Both groups demonstrated accumulation of biological material within the ETT ranging from 0 to 200 μm in the SSD-ETT group and from 50 to 700 μm in St-ETT (Table 2, Fig. 3). No organized biofilm could be documented in any tube; rather, it was common to observe accumulation of secretions with epithelial cells, white and red cells, and bacteria (Table 2).
a, b Confocal scanning laser microscopy: micrographs of the lumen of the ETT at extubation stained with BacLight Live/Dead and imaged with CSLM. a St-ETT sample: cross section is 746 μm thick at its maximum. b SSD-ETT sample: cross section is 64 μm thick. c, d Scanning electron microscopy. c Lumen of St-ETT showing diplococci, macrophages, and epithelial cells on the amorphous deposits. d Lumen of a SSD-ETT. Note absence of any deposit; only a thin layer of silver sulfadiazine can be seen
Discussion
In an in-vitro study, we observed that SSD-ETT have bactericidal properties against Pseudomonas aeruginosa, preventing biofilm formation on the ETT lumen [13]. In sheep intubated and mechanically ventilated, the SSD-ETT was associated with decreased bacterial colonization of the ETT, ventilator circuit, and the lower respiratory tract [13].
This randomized phase I–II clinical trial on cardiac-surgery patients mechanically ventilated for 12–24 h demonstrated that the SSD-ETT was: (1) safely implemented, (2) easy to manage, and (3) decreased significantly the bacterial colonization of the ETT lumen compared to St-ETT.
Medical devices such as intravenous catheters coated with antiseptics or antibiotics have been previously shown to prevent hospital-acquired infections [36, 37]. In this clinical trial, we did not use antibiotics to coat the ETT, in order to avoid increasing dissemination of multiresistant bacteria. In previous laboratory studies [10, 12, 13], we fabricated ten bacteriostatic and bactericidal ETT coatings: (1) chlorhexidine in polyurethane, (2) silver sulfadiazine in polyurethane, (3) silver sulfadiazine and chlorhexidine in polyurethane, (4) silver sulfadiazine and carbon in polyurethane, (5) silver sulfadiazine chlorhexidine and carbon in polyurethane, (6) silver in polyurethane, (7) silver and carbon in polyurethane, (8) silver–platinum in polyurethane, (9) silver–platinum and carbon in polyurethane, and (10) rose bengal for UV light. All of them showed prolonged bacteriostatic and bactericidal effects in vitro and in animal studies. However, we decided to use the silver sulfadiazine in polyurethane coating for this clinical trial because this particular coating is very smooth and resistant to torque and does not require extra care (e. g. UV light), and SSD is already widely and safely used in the clinical setting (e. g. cream preparation, intravenous catheters, urinary catheters, prostheses). Moreover, a recent multicenter study showed that silver-based coating of ETT delayed tracheal aspirate colonization and decreased the bacterial burden of the ETT [38]. A larger clinical trial is now assessing whether such coated ETT may reduce the incidence of VAP.
Our study presents some limitations. First, the study was limited to a 24-h period. While pneumonia can also occur in postoperative patients ventilated for a brief period of time, VAP is defined as pneumonia occurring more than 48 h after endotracheal intubation and initiation of mechanical ventilation [3, 4]. However, our main goal was to prevent bacterial colonization of the ETT, rather than decrease the incidence of VAP. Future studies should evaluate the clinical outcomes of patients with prolonged MV intubated with antiseptic-coated ETT.
Second, the pathogenesis of VAP is multifactorial [3, 4]. Bacteria can enter the lungs through inhalation (through the tube), aspiration (around the cuff), hematogenous spread, and contiguous spread. Thus, preventing bacterial colonization of the ETT might not translate into prevention of VAP. However, previous studies showed that after a few hours of artificial ventilation, colonies of bacteria stick/grow and flourish on the wall of the endotracheal tube [6–9]. These bacteria can be a cause of pneumonia, by (1) aerosolization through the inspiratory gas flow during artificial ventilation, (2) movement of bacteria growing in the lumen of the endotracheal tube into the lungs, or (3) physical translocation of bacteria from the tube into the lungs through the use of a suction catheter. Although ETT are known to form biofilms and to be a large reservoir of microorganisms, preventive strategies are lacking in the clinical setting. The SSD coating proved to prevent colonization of the ETT.
Moreover, in this study we focused on biofilm prevention; however, it might be possible that large amounts of tracheal secretions can still accumulate in the ETT, representing a possible cache for bacteria in patients mechanically ventilated for a prolonged period of time. In their study, Villafane et al. [39] showed that significant alterations in inner ETT configuration occurred in patients ventilated for more than 48 h and suggested that in-vivo monitoring of the lumen of the ETT might be clinically beneficial. This observation is even more important if translated to coated ETT. The antibacterial coating should be in the proximity of the pathogens to prevent colonization. In our experience, the selection of an effective and safe coating is just as important as the complete removal of mucus from the lumen of the ETT [40, 41]. Unlike prostheses or intravenous and urinary catheters, ETT are exposed to constant large amounts of secretions, especially in patients ventilated for prolonged periods. Unfortunately, the standard methods for cleaning ETT via a small, flexible, plastic suction catheter do not remove all secretions. In order to improve the care of intubated and mechanically ventilated patients, coating of the ETT should be combined with novel methods to remove secretions from the ETT.
Conclusion
ETT internally coated with silver sulfadiazine in polyurethane can be easily and safely used in intubated patients and prevent bacterial colonization of the ETT lumen. Further studies should evaluate the clinical outcomes of patients intubated with SSD-ETT and mechanically ventilated for prolonged periods.
References
Vincent JL, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas-Chanoin MH, Wolff M, Spencer RC, Hemmer M (1995) The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. JAMA 274:639–644
National Nosocomial Infections Surveillance System (2004) National Nosocomial Infections Surveillance (NNIS) System Report: data summary from January 1992 through June 2004. Am J Infect Control 32:470–485
Chastre J, Fagon JY (2000) Ventilator-associated pneumonia. Am J Respir Crit Care Med 165:867–903
Hubmayr RD, Burchardi H, Elliot M, Fessler H, Georgopoulos D, Jubran A, Limper A, Pesenti A, Rubenfeld G, Stewart T, Villar J (2002) Statement of the 4th International Consensus Conference in Critical Care on ICU-Acquired Pneumonia – Chicago, Illinois, May 2002. Intensive Care Med 28:1521–1536
Girou E, Schortgen F, Delclaux C, Brun-Buisson C, Blot F, Lefort Y, Lemaire F, Brochard L (2000) Association of noninvasive ventilation with nosocomial infections and survival in critically ill patients. JAMA 284:2361–2367
Sottile FD, Marrie TJ, Prough DS, Hobgood CD, Gower DJ, Webb LX, Costerton JW, Gristina AG (1986) Nosocomial pulmonary infection: possible etiologic significance of bacterial adhesion to endotracheal tubes. Crit Care Med 14:265–270
Inglis TJ, Millar MR, Jones JG, Robinson DA (1989) Tracheal tube biofilm as a source of bacterial colonization of the lung. J Clin Microbiol 27:2014–2018
Adair CG, Gorman SP, Feron BM, Byers LM, Jones DS, Goldsmith CE, Moore JE, Kerr JR, Curran MD, Hogg G, Webb CH, McCarthy GJ, Milligan KR (1999) Implications of endotracheal tube biofilm for ventilator-associated pneumonia. Intensive Care Med 25:1072–1076
Costerton W, Veeh R, Shirtliff M, Pasmore M, Post C, Ehrlich G (2003) The application of biofilm science to the study and control of chronic bacterial infections. J Clin Invest 112:1466–1477
Berra L, De Marchi L, Yu ZX, Laquerriere P, Baccarelli A, Kolobow T (2004) Endotracheal tubes coated with antiseptics decrease bacterial colonization of the ventilator circuits, lungs, and endotracheal tube. Anesthesiology 100:1446–1456
US Food and Drug Administration (1998) US-FDA Public Health Notice: Potential hypersensitivity reactions to chlorhexidine-impregnated medical devices (ed. Burlington B). Available at www.fda.gov/cdrh/chlorhex.html
Berra L, Panigada M, De Marchi L, Greco G, Yu ZX, Baccarelli A, Pohlmann J, Costello KF, Appleton J, Mahar R, Lewandowski R, Ravitz L, Kolobow T (2003) New approaches for the prevention of airway infection in ventilated patients. Lessons learned from laboratory animal studies at the National Institutes of Health. Minerva Anestesiol 69:342–347
Berra L, Curto F, Li Bassi G, Laquerriere P, Pitts B, Baccarelli A, Kolobow T (2008) Antimicrobial-coated endotracheal tubes: an experimental study. Intensive Care Med DOI 10.1007/s00134-008-1099-3
Jarrett F, Ellerbe S, Demling R (1978) Acute leukopenia during topical burn therapy with silver sulfadiazine. Am J Surg 135:818–819
Fraser GL, Beaulieu JT (1979) Leukopenia secondary to sulfadiazine silver. JAMA 241:1928–1929
Wilson P, George R, Raine P (1986) Topical silver sulphadiazine and profound neutropenia in a burned child. Burns Incl Therm Inj 12:295–296
Fraser GL, Beaulieu JT (1979) Leukopenia secondary to sulfadiazine silver. JAMA 241:1928–1929
Hoffmann S (1984) Silver sulfadiazine: an antibacterial agent for topical use in burns. A review of the literature. Scand J Plast Reconstr Surg 18:119–126
Gbaanador GB, Policastro AJ, Durfee D, Bleicher JN (1987) Transient leukopenia associated with topical silver sulfadiazine in burn therapy. Nebr Med J 72:83–85
Choban PS, Marshall WJ (1987) Leukopenia secondary to silver sulfadiazine: frequency, characteristics and clinical consequences. Am Surg 53:515–517
Fuller FW, Engler PE (1988) Leukopenia in non-septic burn patients receiving topical 1% silver sulfadiazine cream therapy: a survey. J Burn Care Rehabil 9:606–609
Thomson PD, Moore NP, Rice TL, Prasad JK (1989) leukopenia in acute thermal injury: evidence against topical silver sulfadiazine as the causative agent. Burn Care Rehabil 10:418–420
Viala J, Simon L, Le Pommelet C, Philippon L, Devictor D, Huault G (1997) Agranulocytosis after application of silver sulfadiazine in a 2-month old infant. Arch Pediatr 4:1103–1106
Kiker RG, Carvajal HF, Mlcak RP, Larson DL (1977) A controlled study of the effects of silver sulfadiazine on white blood cell counts in burned children. J Trauma 17:835–836
Blangy H, Simon D, Levy-Cloez A, Feillet F, Fyad JP, Trechot P, Gillet P,Lascombes P (2002) Topic silver sulfadiazine bicytopenia: first case. Therapie 57:307–309
Eldad A, Neuman A, Weinberg A, Benmeir P, Rotem M, Wexler MR (1991) Silver sulphadiazine-induced haemolytic anaemia in a glucose-6-phosphate dehydrogenase-deficient burn patient. Burns 17:430–432
Jia XM (1985) Hypersensitivity due to topical use of silver sulfadiazine on burn wounds (report of 6 cases) Zhonghua Zheng Xing Shao Shang Wai Ke Za Zhi 1:232–233
McKenna SR, Latenser BA, Jones LM, Barrette RR, Sherman HF, Varcelotti JR (1995) Serious silver sulphadiazine and mafenide acetate dermatitis. Burns 21:310–312
Fraser-Moodie A (1992) Sensitivity to silver in a patient treated with silver sulphadiazine (Flamazine). Burns 18:74–75
Lockhart SP, Rushworth A, Azmy AA, Raine PA (1983) Topical silver sulphadiazine: side effects and urinary excretion. Burns Incl Therm Inj 10:9–12
Fisher NM, Marsh E, Lazova R (2003) Scar-localized argyria secondary to silver sulfadiazine cream. J Am Acad Dermatol 49:730–732
Eldad A, Icekson M, Zur T, Slosser D, Maly A, Arielli D, Burvin R, Ad-El D,Neuman A (2003) Silver-sulfadiazine eschar pigmentation mimics invasive wound infection: a case. J Burn Care Rehabil 24:154–157
Dupuis LL, Shear NH, Zucker RM (1985) Hyperpigmentation due to topical application of silver sulfadiazine cream. J Am Acad Dermatol. 12:1112–1114
Payne CM, Bladin C, Colchester AC, Bland J, Lapworth R, Lane D (1992) Argyria from excessive use of topical silver sulphadiazine. Lancet 340:126
Jennings LJ, Hanumadass M (1988) Silver sulfadiazine induced Clostridium difficile toxic megacolon in a burn patient: case report. Burns 24:676–679
O'Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, Masur H, McCormick RD, Mermel LA, Pearson ML, Raad II, Randolph A, Weinstein RA (2002) Guidelines for the prevention of intravascular catheter-related infections. Centers for Disease Control and Prevention. MMWR Recomm Rep 51:1–29
Johnson JR, Kuskowski MA, Wilt TJ (2006) Systematic review: antimicrobial urinary catheters to prevent catheter-associated urinary tract infection in hospitalized patients. Ann Intern Med 144:116–126
Rello J, Kollef M, Diaz E, Sandiumenge A, del Castillo Y, Corbella X, Zachskorn R (2006) Reduced burden of bacterial airway colonization with a novel silver-coated endotracheal tube in a randomized multiple-center feasibility study. Crit Care Med 34:2766–2772
Villafane MC, Cinnella G, Lofaso F, Isabey D, Harf A, Lemaire F, Brochard L (1996) Gradual reduction of endotracheal tube diameter during mechanical ventilation via different humidification devices. Anesthesiology 85:1341–1349
Kolobow T, Li Bassi G, Curto F, Zanella A (2006) The Mucus Slurper: A novel tracheal tube that requires no tracheal tube suctioning. A preliminary report. Intensive Care Med 32:1414–1418
Berra L, Curto F, Li Bassi G, Laquerriere P, Baccarelli A, Kolobow T (2006) Antibacterial-coated tracheal tubes cleaned with the Mucus Shaver: a novel method to retain long-term bactericidal activity of coated tracheal tubes. Intensive Care Med 32:888–893
Acknowledgements
We thank Hui Zheng PhD, Biostatistics Center, MGH, Boston for statistical review of our results.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is discussed in the editorial available at: http://dx.doi.org/10.1007/s00134-008-1101-0.
Rights and permissions
About this article
Cite this article
Berra, L., Kolobow, T., Laquerriere, P. et al. Internally coated endotracheal tubes with silver sulfadiazine in polyurethane to prevent bacterial colonization: a clinical trial. Intensive Care Med 34, 1030–1037 (2008). https://doi.org/10.1007/s00134-008-1100-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-008-1100-1