Abstract
Objective
To assess the influence of antibiotics on the value of various cytological parameters, and their combinations, in diagnosing ventilator-associated pneumonia (VAP).
Design
Prospective study.
Setting
The general intensive care unit (17 beds) of the University Hospital Maastricht.
Patients
Three hundred and thirty-five episodes of clinically suspected VAP (defined by the clinical and radiological criteria previously described by Bonten et al.) in 282 patients were studied.
Interventions
No additional interventions were conducted.
Measurements and results
Bronchoalveolar lavage fluid cytology included a total cell count per millilitre, differential cell count and the percentage of infected cells (cells containing phagocytised organisms). Antibiotic therapy from 72 h prior to lavage was recorded. Areas under the curve (AUCs) of receiver operating characteristic curves were calculated for various cytological parameters and their combinations, in patients with and without antibiotic therapy. In 126 episodes (37.6%) in 106 patients, VAP was confirmed. There was no difference in AUCs between patients with and without antibiotic therapy for any parameter studied. The most prominent AUCs were (for patient groups with and without antibiotics combined): total cell count, 0.65; percentage polymorphonuclear neutrophils, 0.71; and percentage infected cells, 0.90. The combination of percentage infected cells with any other cytological parameter did not increase the AUC.
Conclusion
Antibiotic therapy did not influence the predictive value of the percentage infected cells in BALF in diagnosing VAP.
Similar content being viewed by others
Introduction
Ventilator-associated pneumonia (VAP) is a common complication in intensive care (ICU) patients, represents up to 80% of the nosocomial infections in the ICU [1, 2] and is associated with high mortality rates [1–3].
Bronchoalveolar lavage (BAL) with quantitative culture is currently regarded as a reference method for the microbiologically confirmed diagnosis of VAP [4] since clinical presentation, radiology and laboratory parameters are non-specific. However, results of BAL fluid (BALF) cultures can take up to 48 h. Because microscopic examination of BALF can be achieved within 2 h [5, 6], the diagnostic value of cytological parameters has been explored. Of the previously investigated parameters, such as the total cell count (TCC) [7–9], the percentage of polymorphonuclear neutrophils (PMNs) [8, 10] and the percentage of cells containing phagocytised organisms, also known as infected cells or cells with intracellular organisms (IC) [6, 11, 12], the last proved to be the most reliable. However, few studies on the influence of antibiotic therapy on the percentage of IC have been published [13–15]. The previously performed studies used a relatively small study population [13], used different techniques for calculation of the percentage IC and had conflicting results [13–15]. Therefore, we set out to evaluate the influence of antibiotic therapy on the predictive value of combinations of cytological parameters of BALF in diagnosing VAP.
Materials and methods
BALF inclusion and laboratory processing
This prospective study was conducted at the University Hospital Maastricht during a 61-month period (from January 1999 to February 2004). The hospital has a general ICU of 17 beds. In this period all consecutive BALF samples of ICU patients with clinical suspicion of (a new episode of) VAP were included. Clinical suspicion of VAP was defined by the clinical and radiological criteria previously described by Bonten et al., i.e. rectal temperature > 38°C or < 35.5°C, blood leukocytosis (> 10 × 103/mm3) and/or left shift or blood leukopenia (< 3 × 103/mm3), more than ten leukocytes per high-power field in Gram stain of tracheal aspirate, positive culture of tracheal aspirate and a new, persistent, or progressive infiltrate on chest radiography [16].
Sampling technique
The affected lung was determined by the radiological presence of infiltrate; if no apparent infiltrate was present, the lingula was lavaged [17]. A fibreoptic bronchoscope (Pentax FB-15H/FB-15X, Pentax Medicals, Tokyo, Japan) was introduced and “wedged” into the affected segmental or subsegmental bronchus. Sterile saline (0.9% NaCl, room temperature) was instilled in four aliquots of 50 ml, immediately aspirated and recovered. BALF samples were transported to the laboratory within 15 min after collection and processed immediately upon arrival at the laboratory.
Laboratory processing
The first fraction of BALF (bronchial fraction) was processed separately for mycobacterial analysis and the three remaining fractions were pooled for further analysis.
BALF samples were quantitatively cultured for bacteria and yeast using 2-μl and 10-μl pipettes [18]. After a total of 48 h, bacterial colonies were counted and identified according to standard methods and minimal inhibitory concentration (MIC) values for most commonly used antimicrobial agents were determined. Cytocentrifuged preparations were made using Cytospin 3 apparatus (Thermo Electron's Anatomical Pathology Group, Astmoor, England) using a standardised protocol: speed 650 rpm, time 10 min, acceleration rate low. A differential cell count was done on May–Grünwald–Giemsa (MGG) stained preparations by counting 500 nucleated cells and identifying; alveolar macrophages (AMs), lymphocytes (Lym), polymorphonuclear neutrophils (PMNs), eosinophils (Eos) and mast cells (MC). IC were expressed as a percentage of the 500 nucleated cells counted [19]. The percentages of squamous epithelial cells and ciliated epithelial cells were counted on 500 nucleated cells and separately noted.
Rejection criteria
BALF samples were rejected if they fitted one of the following criteria: (a) recovered volume less than 20 ml; (b) TCC less than 60,000 cells/ml [9]; (c) presence of excessive amounts of intercellular debris or damaged nucleated cells [5]; (d) presence of more than 1% squamous epithelial cells [20, 21].
Definitions
BALF samples were defined as microbiologically confirmed VAP (VAP group) if the culture yielded organisms in quantities ≥ 104 colony forming units (cfu)/ml. The remaining samples were categorised as non-VAP group. An episode was defined as the 14-day period starting at the time of BAL. For the percentage IC a cut-off value of 2% was used [11].
Antibiotic use
Antibiotic use was recorded for each episode, starting 72 h before BAL and including the day of BAL. We divided patients into two groups: (1) patients with antibiotic therapy and (2) patients without antibiotic therapy. The first group included patients on antibiotic therapy for more than 24 h prior to bronchoscopy. The second group included patients with no antibiotic therapy in the 72 h prior to bronchoscopy, those who were on antibiotic therapy for less than 24 h and those who received antibiotics that were not active against the isolated pathogen.
Statistical analysis
To compare the different cytological parameters for the VAP group versus the non-VAP group, p values were calculated using a Student t-test. To ascertain the value of different cells in diagnosing VAP, in both groups, receiver operating characteristic (ROC) curves were plotted for prediction of VAP using the different cell types and their combinations [22].
Results
Numbers of samples, demographic data and microbiologic findings
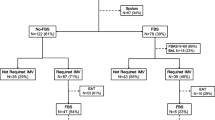
During the study period, 394 consecutive BALF samples were obtained from 394 episodes of suspected VAP in 316 patients. Fifty-nine (14.9%) samples were rejected, leaving 335 samples obtained from 282 patients for inclusion. Figure 1 shows an algorithm for the patients included in the study. Table 1 shows the baseline characteristics of the study patients. The median age of the patients was 60 years (range 15–87 years), and the male-to-female ratio was 2.2:1. A total of 185 (55.2%) episodes were included from 166 patients on antibiotics at the time of bronchoscopy; the remaining 150 (44.8%) episodes occurred in 138 patients who were not on antibiotics at the time of bronchoscopy. In 23 patients with multiple episodes of suspected VAP at least one episode of suspected VAP was whilst the patient was receiving antibiotic therapy and at least one without. VAP was microbiologically confirmed using culture as a reference test in 126 (37.6%) episodes in 106 patients. In 40.5% (51/126) of these episodes, the patient was already receiving antibiotics at the time of the BAL. Table 2 gives an overview of the antibiotics used and whether mono-therapy or combination therapy was given up to 72 h before BAL.
Cytological data
The TCC, the percentages of PMNs, IC in PMNs, IC in AMs and IC (total) were significantly higher in the VAP group, whilst the percentages of AMs, Lym and Eos were significantly higher in the non-VAP group. In 186 samples, IC were observed: in 151 samples (81.2%) only PMNs with phagocytised micro-organisms were found, in 2 samples (1.0%) only AMs with micro-organisms, and in 33 (17.8%) the IC comprised both PMNs and AMs.
Predictive value of cytological parameters
Table 3 shows the predictive value of the various cytological parameters for the diagnosis of VAP, expressed as the AUC of their ROC curves. The TCC, the percentage PMNs and the percentage IC reached the highest AUC values. Combinations of these parameters, however, did not result in an improvement of AUC, nor did any combination of other cytological parameters (Table 3). Antibiotic therapy in 72 h prior to performance of BAL did not influence the predictive value of the IC for the diagnosis of VAP (Table 3). The highest AUC was reached by the percentage IC (PMNs and AMs combined) as a single parameter. Antibiotic therapy in 72 h prior to performance of BAL did not influence the predictive value of the IC for the diagnosis VAP.
Additional data on false-negative and false-positive results
Table 4 shows the quantitative culture results versus the infected cell count. Based on an IC count below the 2% cut-off value, 26 BALF samples of the VAP group (20.6%) were incorrectly assigned to the non-VAP group. In 50% of these patients (13/26), BAL was performed whilst the patient was already receiving antibiotic treatment. Most (17/26, 65.4%) of these false-negative samples showed culture results around the 104 cfu/ml threshold. The micro-organisms involved did not differ from those recovered in the true-positive group (Table 5), and 6 out of 7 micro-organisms recovered were susceptible to the antibiotic the patients was receiving at the time of the BAL. In 17 (65.4%) of these false-negative samples, the MGG stained preparations revealed mucus plugs compiled of PMNs and micro-organisms. The mucus plugs were irregularly distributed over the cytospin spot, and in seven samples infected cells were observed within these plugs. Bacteria cultured most often from samples containing these plugs were Pseudomonas aeruginosa (8/17, 47.1%) and Staphylococcus aureus (2/17, 11.8%). There was no significant difference in the percentages of squamous epithelial cells and bronchial epithelial cells between the false-negative samples and the true-negative samples.
A total of 209 (62.4%) BALF samples had a quantitative culture below 104 cfu/ml (Table 4). Of these samples 16 (7.7%) showed ≥ 2% infected cells and were therefore considered as false-positive results. Their median percentage IC was 3.4% (range 2.0–35.0%) and in six samples, the percentage IC exceeded 5% (range 6.4–35.0%). In half of the patients (8/16) with false-positive results, antibiotic therapy was started before BAL was performed. In four out of eight samples from patients already on antibiotic therapy, BAL yielded a micro-organism susceptible to the given antibiotic therapy. One sample yielded no growth and the remaining three samples yielded bacteria resistant to the antibiotic therapy. Thirteen (13/16, 81.3%), of the false-positive samples reached a quantitative culture result just below the cut-off value of 104 cfu/ml.
Borderline culture results
Of the 335 BALF samples obtained, 225 (67%) yielded one or more micro-organisms (Table 4). A large proportion (139/225, 61.7%) showed quantitative culture results approaching the cut-off value: quantitative culture results of 103 cfu/ml and 104 cfu/ml were found in respectively 83 and 55 samples. There was an equal distribution over episodes with and without antibiotic therapy (74/185, 40.0% vs. 65/150, 43.4%). Lowering the cut-off value for the quantitative culture from 104 cfu/ml to 103 cfu/ml for episodes with antibiotic therapy resulted in a large increase in false-negative infective cell counts. The sensitivity decreased from 79.4% (95% CI 75.4–83.4%) to 60.6% (95% CI 57.1–64.1%) with a slight increase in specificity from 92.3% (95% CI 88.0–96.6%) to 93.8% (95% CI 89.4–98.1%).
Discussion
The main, and clinically relevant, finding of the present study is that antibiotic therapy in the period of 72 h prior to performance of BAL under suspicion of VAP did not influence the reliability of the infected cell count in BALF in the prediction of microbiologically confirmed VAP. Among the cytological parameters of BALF cytocentrifuged preparations, the percentage IC most accurately predicted the presence of VAP. Moreover, combining percentage IC with any other cytological parameters did not increase the predictive value for VAP.
The microscopic evaluation of the percentage of IC in BALF has proven to be a reliable tool in the diagnosis of VAP [6, 8, 9, 13, 15, 23, 24]. However, there are large differences in the methodology and in the cut-off values used in these different studies, making comparison of the study results difficult. Furthermore, conflicting results have been published concerning antibiotic influence on this parameter. The method used in this study was highly standardised [19]. Three parameters (TCC, percentage PMNs and percentage IC) were found to have significantly higher values in patients with VAP than in the non-VAP group. Of these, the AUC for percentage IC appeared to display the greatest difference. Combining the different cytological parameters, however, did not improve the predictive value in diagnosing VAP. This can be explained by the high AUC of the percentage IC on its own, as well as by the number of other clinical conditions that cause elevations of the percentage PMNs in ventilated patients [25]. No difference was found in the predictive value of cytological parameters between patients with and without antibiotic therapy at the time of the BAL (started up to 72 h before). This is in contrast with previous studies [13, 15]. Dotson et al. found that both current antibiotic use and a recent history of antibiotic use negatively influenced the recovery of IC. In their group of seven patients with VAP already on antibiotic therapy before bronchoscopy, they found only two patients (28%) with IC in BALF. Furthermore, the micro-organisms recovered from these two patients were resistant to the antibiotic regime administered. In our study, 74.5% (38/51) of episodes of VAP were correctly predicted by IC in the group receiving antibiotics. This significant difference could in part be explained by the size of the research group. Dotson et al. investigated 49 episodes (31 without antibiotic therapy, 18 with recent antibiotic therapy), whereas our study included 335 episodes of VAP. A second possible explanation is the cut-off value they used for IC, namely 7%. In the present study 2% IC yielded the best predictive value; with higher cut-off values the sensitivity declined and specificity increased. Sirvent et al. [15] evaluated protected bronchoalveolar mini-lavage fluid and found a decline in sensitivity and specificity of IC for the prediction of VAP in patients receiving antibiotic therapy up to 72 h before BAL. Moreover, Sirvent et al. [15] found a high percentage (16/65, 22.2%) of Haemophilus influenzae as causative organisms in their patients with VAP, compared to 12.6% (16/126) in the present study. Since H. influenzae, due to its small size and fragile appearance, can easily be missed in both MGG- and Gram-stained preparations, we hypothesised that this could attribute to the difference in sensitivity between the two studies. However, the authors did not mention the organisms involved in the false-negative results [15]. The findings of the present study are in line with those of Timsit et al. [14], who found no difference in sensitivity and specificity of the IC count between patients with and without antibiotic therapy in the 48 h before BAL.
The presence of plugs containing numerous bacteria may explain in part the false-negative samples. As these samples did not contain high numbers of squamous and/or bronchial epithelial cells, these plugs did not represent bronchial or oropharyngeal contamination. In histological studies, it has been shown that early stages of pneumonia are associated with focal accumulation of PMNs in the smaller bronchi, with spreading of PMNs throughout the lung in advanced pneumonia [26]. Hence, we hypothesised that the observed plugs were initial foci of pneumonia and should be actively looked for in the low-power field setting (magnification 100×). An alternative explanation may be the quantitative culture threshold used for defining VAP: the cut-off value of 104 cfu/ml was chosen in order to prevent failure to diagnose VAP and consequently may have overestimated the frequency of VAP [27]. In this regard, it is interesting that nearly two thirds of false-negative samples had quantitative cultures close to the 104 cfu/ml threshold. It is possible that some of the culture-positive samples with less than 2% IC should actually be considered as non-VAP. Some authors have suggested decreasing the cut-off value to 103 in the case of antibiotic therapy in the preceding 72–24 h of lavage [28, 29]. The present study does not support this view since no significant difference was found in the distribution of quantitative culture results (excluding the episodes which did not yield any bacteria in BALF) between patients with and those without antibiotic therapy. Furthermore, the sensitivity for IC in predicting VAP decreased from almost 80% to 60%, with only a slight increase in specificity.
There was no obvious explanation for the 7.7% false-positive results. Irrespective of the use of antibiotics, the high percentage of IC in many of the false-positive samples, however, gives rise to concern with regard to the exclusion of VAP when the culture threshold is not reached. In this respect, it is interesting that recent studies define VAP not only by using the 104 cfu/ml culture threshold but also by incorporating the 2% cut-off value of IC as a criterion independently of the culture threshold. This approach has also been implemented in daily practice in our ICU [24, 30–34]. Supportive of this approach is the fact that more than 80% of the false positives reached a quantitative culture result just below the cut-off value. Using the above-cited combination of criteria to define VAP (i.e. combining the 104 cfu/ml culture threshold and the 2% IC value), the number of VAP episodes in the present study would have increased from 126/335 (37.6%) to 137/335 (41.0%).
A limitation of the presented study is the lack of a gold standard for the diagnosis VAP. Instead, a “semi-gold” standard, namely the quantitative culture of BALF, was used. Unfortunately, this “semi-gold standard” itself is influenced by antibiotic therapy. Therefore, it was only possible to study the influence of antibiotic therapy on the infected cell count in microbiologically confirmed VAP (with quantitative cultures exceeding the cut-off value of 104 cfu/ml) (since autopsy as a gold standard is not practically useful).
In the present study neither the occurrence of other conditions associated with influx of PMNs in the IC population nor the episodes with borderline quantitative culture results were studied. Therefore, an additional study is required to address the presence of qualitative cytological parameters suggesting non-infectious conditions, such as eosinophils, foamy alveolar macrophages, activated lymphocytes, plasma cells and reactive type II pneumocytes (RPII cells) [35]. The presence of RPII cells has been associated with severe alveolar damage. A prospective evaluation of cytological parameters in different non-infectious conditions should be established to enable the construction of a predictive computer model in line with that constructed for use in the assessment of interstitial lung diseases [36].
The present findings are relevant for ICUs in other hospitals, since the study population represents a heterogeneous group of intensive care patients comparable to that seen in general ICUs in many other institutions. The presently used stains and techniques are within the scope of any microbiology laboratory. If the MGG stain is not available, laboratories may fall back on the Gram stain, as the latter also enables reliable detection of the percentage IC in BALF samples [19]. Since the percentage IC encompasses both PMNs and AMs, no distinction between these cell types is required, precluding the need for more specialised cytological expertise on the part of the laboratory staff.
In conclusion, antibiotic therapy in the 72 h preceding the BAL did not influence the predictive value of cytological BALF parameters in the microscopic diagnosis of VAP. The percentage of IC appears to be the most important parameter for distinguishing VAP from non-VAP conditions. Combining the percentage IC with any other cytological parameter did not result in better predictive values.
References
Chastre J, Fagon JY (2002) Ventilator-associated pneumonia. Am J Respir Crit Care Med 165:867–903
American Thoracic Society, Infectious Diseases Society of America (2005) Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171:388–416
National Nosocomial Infections Surveillance (NNIS) (1999) System report, data summary from January 1990–May 1999, issued June 1999. Am J Infect Control 27:520–532
Grossman RF, Fein A (2000) Evidence-based assessment of diagnostic tests for ventilator-associated pneumonia. Executive summary. Chest 117[4 Suppl 2]:177S–181S
Jacobs JA, De Brauwer E (1999) BAL fluid cytology in the assessment of infectious lung disease. Hosp Med 60:550–555
Timsit JF, Cheval C, Gachot B, Bruneel F, Wolff M, Carlet J, Regnier B (2001) Usefulness of a strategy based on bronchoscopy with direct examination of bronchoalveolar lavage fluid in the initial antibiotic therapy of suspected ventilator-associated pneumonia. Intensive Care Med 27:640–647
Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew PD, Suter PM (1991) Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis 143:1121–1129
Allaouchiche B, Jaumain H, Dumontet C, Motin J (1996) Early diagnosis of ventilator-associated pneumonia. Is it possible to define a cutoff value of infected cells in BAL fluid? Chest 110:1558–1565
Timsit JF, Misset B, Goldstein FW, Vaury P, Carlet J (1995) Reappraisal of distal diagnostic testing in the diagnosis of ICU-acquired pneumonia. Chest 108:1632–1639
Sole-Violan J, Rodriguez de Castro F, Rey A, Martin-Gonzalez JC, Cabrera-Navarro P (1994) Usefulness of microscopic examination of intracellular organisms in lavage fluid in ventilator-associated pneumonia. Chest 106:889–894
Allaouchiche B, Jaumain H, Chassard D, Bouletreau P (1999) Gram stain of bronchoalveolar lavage fluid in the early diagnosis of ventilator-associated pneumonia. Br J Anaesth 83:845–849
Veber B, Souweine B, Gachot B, Chevret S, Bedos JP, Decre D, Dombret MC, Dureuil B, Wolff M (2000) Comparison of direct examination of three types of bronchoscopy specimens used to diagnose nosocomial pneumonia. Crit Care Med 28:962–968
Dotson RG, Pingleton SK (1993) The effect of antibiotic therapy on recovery of intracellular bacteria from bronchoalveolar lavage in suspected ventilator-associated nosocomial pneumonia. Chest 103:541–546
Timsit JF, Misset B, Renaud B, Goldstein FW, Carlet J (1995) Effect of previous antimicrobial therapy on the accuracy of the main procedures used to diagnose nosocomial pneumonia in patients who are using ventilation. Chest 108:1036–1040
Sirvent JM, Vidaur L, Gonzalez S, Castro P, de Batlle J, Castro A, Bonet A (2003) Microscopic examination of intracellular organisms in protected bronchoalveolar mini-lavage fluid for the diagnosis of ventilator-associated pneumonia. Chest 123:518–523
Bonten MJ, Bergmans DC, Stobberingh EE, van der Geest S, De Leeuw PW, van Tiel FH, Gaillard CA (1997) Implementation of bronchoscopic techniques in the diagnosis of ventilator-associated pneumonia to reduce antibiotic use. Am J Respir Crit Care Med 156:1820–1824
Haslam PL, Baughman RP (1999) Report of ERS Task Force: guidelines for measurement of acellular components and standardization of BAL. Eur Respir J 14:245–248
Jacobs JA, De Brauwer EI, Cornelissen EI, Drent M (2000) Accuracy and precision of quantitative calibrated loops in transfer of bronchoalveolar lavage fluid. J Clin Microbiol 38:2117–2121
De Brauwer E, Jacobs J, Nieman F, Bruggeman C, Drent M (1999) Test characteristics of acridine orange, Gram, and May–Grunwald–Giemsa stains for enumeration of intracellular organisms in bronchoalveolar lavage fluid. J Clin Microbiol 37:427–429
Aubas S, Aubas P, Capdevila X, Darbas H, Roustan JP, Du Cailar J (1994) Bronchoalveolar lavage for diagnosing bacterial pneumonia in mechanically ventilated patients. Am J Respir Crit Care Med 149:860–866
Gerbeaux P, Ledoray V, Boussuges A, Molenat F, Jean P, Sainty JM (1998) Diagnosis of nosocomial pneumonia in mechanically ventilated patients: repeatability of the bronchoalveolar lavage. Am J Respir Crit Care Med 1571:76–80
Obuchowski NA, Lieber ML, Wians FH Jr (2004) ROC curves in clinical chemistry: uses, misuses, and possible solutions. Clin Chem 50:1118–1125 Epub 2004 May 13
Chastre J, Fagon JY, Soler P, Bornet M, Domart Y, Trouillet JL, Gilbert C, Hance AJ (1988) Diagnosis of nosocomial bacterial pneumonia in intubated patients undergoing ventilation: comparison of the usefulness of bronchoalveolar lavage and the protected specimen brush. Am J Med 85:499–506
Chastre J, Trouillet JL, Vuagnat A, Joly-Guillou ML, Clavier H, Dombret MC, Gilbert C (1998) Nosocomial pneumonia in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 157:1165–1172
Jacobs JA, De Brauwer EI, Ramsay G, Cobben NA, Wagenaar SS, van der Ven AJ, Bruggeman CA, Drent M (1999) Detection of non-infectious conditions mimicking pneumonia in the intensive care setting: usefulness of bronchoalveolar fluid cytology. Respir Med 93:571–578
Corley DE, Kirtland SH, Winterbauer RH, Hammar SP, Dail DH, Bauermeister DE, Bolen JW (1997) Reproducibility of the histologic diagnosis of pneumonia among a panel of four pathologists: analysis of a gold standard. Chest 112:458–465
Meduri GU, Chastre J (1992) The standardization of bronchoscopic techniques for ventilator-associated pneumonia. Chest 102[5 Suppl 1]:557S–564S
Wermert D, Marquette CH, Copin MC, Wallet F, Fraticelli A, Ramon P, Tonnel AB (1998) Influence of pulmonary bacteriology and histology on the yield of diagnostic procedures in ventilator-acquired pneumonia. Am J Respir Crit Care Med 158:139–147
Baker AM, Bowton DL, Haponik EF (1995) Decision making in nosocomial pneumonia. An analytic approach to the interpretation of quantitative bronchoscopic cultures. Chest 107:85–95
Trouillet JL, Chastre J, Vuagnat A, Joly-Guillou ML, Combaux D, Dombret MC, Gilbert C (1998) Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am J Respir Crit Care Med 157:531–539
Combes A, Figliolini C, Trouillet JL, Kassis N, Wolff M, Gibert C, Chastre J (2002) Incidence and outcome of polymicrobial ventilator-associated pneumonia. Chest 121:1618–1623
Hayon J, Figliolini C, Combes A, Trouillet JL, Kassis N, Dombret MC, Gilbert C, Chastre J (2002) Role of serial routine microbiologic culture results in the initial management of ventilator-associated pneumonia. Am J Respir Crit Care Med 165:41–46
Delclaux C, Roupie E, Blot F, Brochard L, Lemaire F, Brun-Buisson C (1997) Lower respiratory tract colonization and infection during severe acute respiratory distress syndrome: incidence and diagnosis. Am J Respir Crit Care Med 156:1092–1098
Marquette CH, Copin MC, Wallet F, Neviere R, Saulnier F, Mathieu D, Durocher A, Ramon P, Tonnel AB (1995). Diagnostic tests for pneumonia in ventilated patients: prospective evaluation of diagnostic accuracy using histology as a diagnostic gold standard. Am J Respir Crit Care Med 151:1878–1888
Jacobs JA, Dieleman MM, Cornelissen EI, Groen EA, Wagenaar SS, Drent M (2001) Bronchoalveolar lavage fluid cytology in patients with Pneumocystis carinii pneumonia. Acta Cytol 45:317–326
Drent M, Jacobs JA, Cobben NA, Costabel U, Wouters EF, Mulder PG (2001) Computer program supporting the diagnostic accuracy of cellular BALF analysis: a new release. Respir Med 95:781–786
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Linssen, C.F.M., Jacobs, J.A., Schouten, J.S.A.G. et al. Influence of antibiotic therapy on the cytological diagnosis of ventilator-associated pneumonia. Intensive Care Med 34, 865–872 (2008). https://doi.org/10.1007/s00134-008-1015-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-008-1015-x