Abstract
Objective
To avoid ventilator induced lung injury, tidal volume should be low in acute lung injury (ALI). Reducing dead space may be useful, for example by using a pattern of inspiration that prolongs the time available for gas distribution and diffusion within the respiratory zone, the mean distribution time (MDT). A study was conducted to investigate how MDT affects CO2 elimination in pigs at health and after ALI.
Design and setting
Randomised crossover study in the animal laboratory of Lund University Biomedical Center.
Subjects and intervention
Healthy pigs and pigs with ALI, caused by surfactant perturbation and lung-damaging ventilation were ventilated with a computer-controlled ventilator. With this device each breath could be tailored with respect to insufflation time and pause time (T I and T P) as well as flow shape (square, increasing or decreasing flow).
Measurements and results
The single-breath test for CO2 allowed analysis of the volume of expired CO2 and the volume of CO2 re-inspired from Y-piece and tubes. With a long MDT caused by long T I or T P, the expired volume of CO2 increased markedly in accordance with the MDT concept in both healthy and ALI pigs. High initial inspiratory flow caused by a short T I or decreasing flow increased the re-inspired volume of CO2. Arterial CO2 increased during a longer period of short MDT and decreased again when MDT was prolonged.
Conclusions
CO2 elimination can be enhanced by a pattern of ventilation that prolongs MDT. Positive effects of prolonged MDT caused by short T I and decreasing flow were attenuated by high initial inspiratory flow.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During mechanical ventilation, oxygenation can in most instances be maintained at very low alveolar ventilation by increasing the fraction of inspired oxygen. Exchange of CO2, however, depends upon alveolar ventilation. Enhanced CO2 elimination without applying high airway pressure caused by high tidal volume (V T) is an issue in some clinical situations. At acutely increased intracranial pressure a low P aCO2 at low airway pressures may be desired, at least in an initial stage. In acute obstructive lung disease enhanced CO2 elimination at low minute ventilation is often desired. Limitation of pressure and V T is a strategy for lung-protective ventilation in acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) [1–6]. With respect to gas exchange, an optimal benefit from a particular V T is an issue, whatever target values for arterial pH or P aCO2 are chosen.
An optimal pattern of V T delivery may enhance CO2 elimination, and an inspiratory pause may reduce respiratory dead space (V D) or P aCO2 [7–11]. Such positive effects have not always been observed, however [9, 12–14]. Diverging results may reflect methodological limitations.
Capnography in the format of the single-breath test for CO2 (SBT-CO2) allows accurate determination of CO2 elimination and partitions of V D [15]. Uttman et al. showed in healthy pigs that CO2 elimination depends on time available for gas distribution and diffusion within the respiratory zone, mean distribution time (MDT) [16]. Variation of MDT was achieved by changing the duration of the postinspiratory pause (T P). CO2 elimination varied in proportion to the logarithm of MDT. Aboab et al. recently reported similar findings in ARDS patients [17]. In the latter study the concept of MDT was modified by taking into account the time required during inspiration to bring the fresh gas interface down to the respiratory zone. Furthermore, the concept of distribution was widened to include CO2 exchange with alveolar blood. Still, knowledge is limited about how different combinations of duration of inspiratory gas insufflation (T I) and T P affect CO2 elimination and, ultimately, P aCO2.
The objective of the present study in pigs – healthy and after ALI – was to test the hypothesis that MDT describes how variation of inspiratory gas delivery affects breath-by-breath CO2 elimination, when MDT is changed for one breath at a time. The study also explores whether a permanent lowering of MDT would constantly increase P aCO2 and vice versa.
Methods
Material
The local Ethics Board for Animal Research approved the study. Twenty-two pigs of the Swedish native breed, weighing 17–24 kg, were fasted overnight with free access to water. The animals were pre-medicated with azaperone (7 mg · kg−1) and anaesthetised with ketamine (5 mg · kg−1). Anaesthesia was maintained by continuous infusion of fentanyl (60 μg · kg−1 · h−1) and midazolam (0.7 mg · kg−1 · h−1).
ALI/ARDS was induced in 14 pigs by combining surfactant perturbation by inhalation of dioctyl sodium sulphosuccinate with very large V T ventilation [18]. Details can be found in the electronic supplementary material (ESM) and in [19].
Pancuronium (0.5 mg · kg−1 · h−1) was given only to healthy pigs. In ALI pigs, paralysis was avoided in order to allow judgement of anaesthesia depth during the longer experiments. With the anaesthesia practised no muscular movements were observed. Ventilation was maintained using a 7.0 mm ID tracheal tube connected to a ventilator (ServoVentilator 900C, Siemens–Elema, Solna, Sweden). A mainstream analyser (CO2 Analyzer 930, Siemens–Elema, Solna, Sweden) measured partial pressure of CO2 at airway opening (P aoCO2). The ventilator/computer system used for data recording has previously been described [20]. Signals from the ventilator and CO2 analyser representing flow rate, airway pressure and P aoCO2 were sampled at the frequency of 100 Hz. The signals had a 50% response time of 12 ms and were synchronous within ± 8 ms. Compliance of the tracheal tube and ventilator tubing was measured in vitro. The system was tested for leakage.
For all pigs, the ventilator was at baseline set at volume control with square inspiratory flow, T I 33% and T P 10% of the respiratory cycle. Minute ventilation was adjusted to achieve P aCO2 5–6 kPa.
Protocol
Part 1: Inspiratory gas delivery modified for one breath at a time
Eight healthy pigs were, after preparation, stabilised for a period of 60 min at baseline ventilation at a fraction of inspired oxygen (FIO2) of 0.21. To combat the high tendency towards lung collapse in pigs, a positive end-expiratory pressure (PEEP) of 8 cmH2O was used [21]. These pigs were studied at respiratory rate (RR) 20 min−1 and 40 min−1, denoted HealthRR20 and HealthRR40. The non-linear influence of MDT on CO2 exchange was considered to merit a primary exploration of particularly short MDT values at high RR, which may be used to reduce V T in ARDS [19].
Six pigs were studied 24 h after induction of ALI/ARDS. These pigs were part of another study comparing how different modes of ventilation affect lung function (see ESM). After stabilisation at PEEP 10 cm H2O and FIO2 1.0, the effect of inspiratory flow patterns on CO2 elimination of single breaths was studied at RR 20 min−1. This group of pigs was denoted ALIRR20.
For all groups of pigs (HealthRR20, HealthRR40 and ALIRR20) the pattern of inspiratory gas delivery was modified for single breaths at a time, with respect to T I (0.2–1.9 s), T P (0.1–0.5 s) and inspiratory flow wave form (SHAPE), in different combinations. SHAPE was either square, increasing, or decreasing flow rate. The latter two shapes were linear ramps starting or ending at zero flow. V T, PEEP and expiratory time were constant for all breaths. A recording sequence was pre-programmed in the computer that momentarily controlled the ventilator. Every 3rd breath out of 12 breaths comprising a recording sequence was modified. The breath immediately preceding a modified breath was defined as a control breath. Five different recording sequences, each with four modified breaths, gave 20 combinations of changes in T I, T P and SHAPE. In randomised order, the five recording sequences were repeated three times.
Part 2: Prolonged periods of constant pattern of gas delivery
In eight healthy pigs (the same animals as the healthy pigs of part 1) and eight other pigs studied 4 h after induction of ALI/ARDS (ALITp) alternative patterns of inspiratory gas delivery were maintained for prolonged periods in order to study the effect on P aCO2. The reason for using a different group of ALI pigs than those used in part 1 was logistical; the total study time would otherwise have been too long. T P was maintained at 17% for 40 min, then changed to 3%, which setting was again applied during 40 min, and finally the initial 17% was set again and applied also during 40 min. Thereby, during the middle period, MDT was changed by a factor of 0.5, i.e. from 80 ms to 40 ms. A reciprocal change in expiratory time maintained RR unchanged. Also V T was unchanged. P aCO2 was measured every 5 min.
Data analysis
Sampled data of flow, pressure and P aoCO2 were transferred to an Excel workbook (Microsoft, Redmond, WA, USA) and analysed according to Uttman et al. [22]. Tidal CO2 elimination (V TCO2) represents the difference between expired volume of CO2 (V ECO2) and that re-inspired from the Y-piece and adjacent tubing (V ICO2) (Fig. 1). Variations in V ICO2, V ECO2 and V TCO2 resulting from variation of inspiratory pattern were expressed in percentage of average V TCO2 from the four control breaths in the same recording sequence immediately preceding the modified breaths and denoted ΔV ICO2, ΔV ECO2 and ΔV TCO2, respectively.
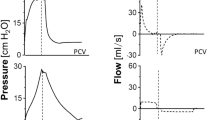
An example of single-breath test for CO2 from a healthy pig. Expired volume of CO2 (V ECO2), is the area under the expiratory curve (grey and hatched areas). Volume of CO2 re-inspired from the Y-piece and adjacent tubing (V ICO2), is shown by the hatched area. The grey area represents tidal elimination of CO2 (V TCO2). When inspiratory gas insufflation was prolonged from 0.75 s to 1.26 s, V ECO2 increased (yellow area) as a consequence of the increase in mean distribution time from 0.51 s to 0.66 s. The decrease in V ICO2 (blue area) was due to the decrease in F early from 0.37 l/s to 0.12 l/s. Accordingly, V TCO2 increased by the sum of the yellow and blue areas
Airway dead space (V Daw) was defined as the point of maximum slope of the SBT-CO2. Over the alveolar plateau, P aoCO2 was described by the equation
where V E is volume of expired gas. Alveolar PCO2 (P ACO2) represents the midpoint of the plateau.
Variation in V ECO2 results from a shift of the ascending limb along the volume axis that reflects a change in V Daw (ΔV Daw) and a shift of the alveolar plateau along the PCO2 axis (ΔP ACO2).
MDT was calculated from flow samples during T I and T P and with respect to V Daw according to Aboab et al. [17]. Flow rate at onset of inspiration (Fearly) was at square inspiratory flow V T/T I, at decreasing flow twice that value, and zero for increasing flow. The sum of T I and T P (TI + P) was calculated as it represents time for transfer of CO2 from blood to alveolar gas.
Statistical methods
Data are presented as mean ± standard deviation (SD), or as mean ± standard error of the mean (SEM) when error of the mean is the issue. Regression analysis was used to study variations in volumes of CO2 in relation to parameters describing inspiratory flow pattern. Student's paired two-tailed t-test was used to analyse changes in P aCO2 during prolonged periods of altered T P.
Results
VT and arterial blood gases are shown in Table 1. The ALIRR20 group was non-homogeneous with respect to gas exchange as reported in ESM.
Part 1
At HealthRR20, HealthRR40 and ALIRR20, V ICO2 for control breaths was 16 ± 1, 34 ± 5 and 12 ± 1% of V ECO2, respectively. ΔV ICO2 showed a significant positive correlation to Fearly that reflects T I and SHAPE (Table 2, Fig. 1).
In breaths with square flow but varying T I and T P, ΔV ECO2 increased significantly at higher MDT at all conditions. A logarithmic relationship was slightly better than a linear relationship for all conditions, but significantly so only for HealthRR20 (p< 0.001) (Table 2, Fig. 2). Regression coefficient d, expressing the influence of lnMDT on ΔV ECO2, was significantly higher at HealthRR40 than at HealthRR20 (p< 0.001). At ALIRR20 d was significantly lower than at HealthRR20 (p< 0.001).
Variations in expired volume of CO2, expressed in percentage of average tidal elimination of CO2 from control breaths (ΔV E CO 2). ΔV ECO2 related to mean distribution time (MDT) for all breaths studied and corresponding regression lines (black lines). Healthy pigs ventilated at low (Health RR20) and high (Health RR40) respiratory rate and pigs after induction of ALI/ARDS (ALI RR20). Breaths with similar total inspiratory time (T I + P) are indicated in a specific colour. Distributions around regression lines were independent of T I + P
At HealthRR20 and ALIRR20, 56–58% of the change in g ΔV ECO2 was caused by ΔV Daw and the remaining 42–44% by ΔP ACO2. At HealthRR40 the contribution to g ΔV ECO2 by ΔV Daw was 69% and by ΔP ACO2 31%.
Differences between measured ΔV ECO2 and values calculated from the logarithmic equations in Table 2 were calculated. No significant correlation between T I + P and these residuals was found for HealthRR20, HealthRR40 or ALIRR20, as can be appreciated from Fig. 2. Accordingly, variations in T I + P had no significant effect upon ΔV ECO2 apart from effects explained by MDT.
At HealthRR40, when MDT was varied by changing SHAPE, ΔV ECO2 varied similarly in relation to MDT as when T I or T P were varied. However, at HealthRR20, ΔV ECO2 varied significantly less when MDT was changed by varying SHAPE. At ALIRR20, no significant effect on ΔV ECO2 was observed when SHAPE was varied. For breaths with square inspiratory flow, ΔV TCO2 varied with lnMDT and F early as shown in Table 2. The coefficients f and g differed significantly between HealthRR20 and each of the conditions HealthRR40 and ALIRR20 (p< 0.001)
Part 2
At HealthRR20 P aCO2 increased at short MDT (Fig. 3). The average of the three last observations during each period of 40 min was considered to represent steady state. At HealthRR20 and ALITp, average P aCO2 increased significantly during the period of short MDT and decreased significantly when MDT was again prolonged (Table 3). The change in P aCO2 was on average 75% of ΔV TCO2 resulting from changing MDT, estimated from equations in Table 2.
Observed P aCO2 when switching from a long to a short postinspiratory pause and back again, resulting in a change in mean distribution time from 0.8 s to 0.4 s. For each pig the values are normalised to the mean value of the three measurements just before shortening the postinspiratory pause. Average ± SEM from six healthy pigs. During each period, the last three observations were used for calculation of data in Table 3
Discussion
The previously described system based upon a computer-controlled ventilator was amended to allow changed pattern of a single inspiration at a time [20]. In part 1, T I, T P and SHAPE were modified while V T, expiratory time and PEEP were unchanged. This allowed a comprehensive analysis of how different patterns of inspiratory flow affect CO2 exchange by using SBT-CO2. By comparing modified breaths with control breaths in the same recording sequence, influence from even minor deviations from a steady state was avoided. The technique for modification of single breaths allowed studies of 21 inspiratory flow patterns in a short time. Uttman et al. introduced the concept of MDT to explain how inspiratory flow pattern affects CO2 exchange by its effect on distribution of inspired gas in the alveolar zone [16]. Aboab et al. stressed that MDT refers not only to time for gas distribution and diffusion within alveolar space, but to time for all phenomena associated with transfer of CO2 from circulating blood in the alveolar capillaries to the airways [17]. This may include movements caused by the heart and pulsating blood.
VICO2 was larger than the volume of CO2 in the Y-piece connecting ventilator tubing to the airway. This reflects mixing of gas in the inspiratory and expiratory lines shown by Fletcher et al. [23]. During the first part of inspiration, while CO2 is present in both inspiratory and expiratory lines, a high flow rate increases turbulence and possibly also Coanda and Bernoulli effects around the Y-piece. This may explain the correlation between Fearly and VICO2. VICO2 amounted to about 16% of VECO2 at HealthRR20 and to 34% at HealthRR40. Re-inspiration of CO2 is considerable. Within a low VT strategy, reduction of VICO2 can be achieved by one-way valves in the Y-piece, as suggested by Fletcher et al., or by aspiration of dead space, as discussed by De Robertis et al. [24].
In accordance with the hypothesis based upon previous studies, ΔV ECO2 increased in relation to lnMDT [16, 17]. A better fit of a logarithmic equation rather than a linear one agrees with concepts based upon physiology and morphology. As gas distribution in lung periphery and exchange with alveolar blood depends on diffusion, gas exchange would be negligible at zero MDT. A very long MDT would imply that the interface between resident alveolar gas and fresh inspired gas is by diffusion brought up to a level at which the summed surface area according to the model of Weibel is so small that further diffusion becomes negligible [25]. When MDT increases from zero to high values, one may accordingly expect a fast initial increase in ΔV ECO2 that becomes ever slower with further MDT increase. Patterns with particularly short MDT may severely reduce gas exchange, as the results at HealthRR40 shows. When increased RR is used in ALI/ARDS in order to limit V T, it may be particularly important to maintain an adequate MDT by prolonging T P and shortening expiration time.
At prolonged MDT, increasing ΔV ECO2 reflected both a decrease of V Daw and a positive ΔP ACO2. This is in line with the results of Aboab et al. [17], who reasoned that a higher level of the alveolar plateau might, at least partially, be explained by continuing delivery of CO2 by alveolar perfusion during a prolonged pause. T I + P represents the time for alveolar perfusion during inspiration. In the present study we found that variation of T I + P by different combinations of T I and T P did not significantly affect ΔV ECO2 above what was explained by MDT. This suggests that time for alveolar perfusion during inspiration is of low importance compared to time for distribution and diffusion within the alveolar zone as expressed by MDT. A possible explanation why T I + P in itself did not affect ΔV ECO2 is that CO2 delivered by alveolar perfusion late during inspiration does not, to a detectable extent, reach the upper respiratory zone in time to be expelled by the ensuing expiration.
A defined change in MDT had a similar effect on ΔV ECO2 regardless of whether the change was caused by varying T I or T P (Fig. 2). Mathematical analysis shows that for a given increase in T P the effect on MDT is three times larger than a comparable increase in T I (see ESM). Accordingly, it is much more efficient to prolong T P than T I.
The finding that at HealthRR20 and at ALIRR20, ΔV ECO2 varied less and even insignificantly when MDT was changed by varying SHAPE implies that variation of SHAPE has effects on gas exchange that do not relate only to MDT. From studies based on flow oscillation techniques we know that sudden flow transients at airway opening lead to oscillations at frequencies around 5 Hz throughout the respiratory system. A hypothetical explanation for a maintained ΔV ECO2 at increasing flow in spite of a shorter MDT is the following: At increasing flow the sudden end-inspiratory flow cessation leads to enhanced diffusion by way of important oscillations in lung periphery. Correspondingly, at decreasing flow, absence of end-inspiratory oscillations may explain why CO2 elimination was lower than expected on the basis of prolonged MDT at this SHAPE.
In part 2, when MDT was varied for periods of 40 min, P aCO2 was expected to approach a steady state while CO2 stores in the body became equilibrated [26, 27]. During the periods of changed T P and thereby changed MDT, P aCO2 changed in the direction expected. The effect of reduction of T P and MDT was less marked in ALITp than in HealthRR20, but not significantly so. We do not speculate about the reasons for the possible difference observed. The change in P aCO2 was, for both HealthRR20 and ALITp, 75% of the change in V TCO2 estimated by using the equations in Table 2. In man, the time constant for the change in P aCO2 was 35 min when ventilation was decreased [27]. Incomplete steady state partially explains why the change in P aCO2 was < 100% of predicted change in V TCO2. The period of changed T P was limited to 40 min in order to limit interference from unavoidable changes in metabolism and other physiological factors affecting CO2 exchange during prolonged experiments. Apart from incomplete steady state there may be other reasons why P aCO2 did not change as much as estimated change in V TCO2. For example, increasing intrapulmonary PCO2 may affect distribution of pulmonary ventilation and perfusion or may change bronchial tone and thereby modify CO2 elimination.
In HealthRR20 the regression coefficient d was somewhat higher than in the non-homogeneous ALIRR20 group, indicating that an increase in MDT results in a slightly higher increase in CO2 elimination in healthy pigs than in pigs after ALI. The present findings are comparable to observations in ARDS patients [17]. It appears that the absence of collateral ventilation in pigs has limited importance with respect to effects of MDT [28]. This is in line with the concept that a longer MDT promotes gas exchange by allowing more time for diffusion of gases in the border zone between conductive airways and the respiratory zone of the lung. Selection of an inspiration pattern providing a longer MDT can be made only in patients who are not breathing spontaneously. The improvement in gas exchange caused by a pattern optimising MDT may lead to reduction of V T in the range of not more than 5–8% at ordinary RR. The non-linear relationship between CO2 exchange and MDT implies that MDT becomes more important at increased rates. One should see optimisation of MDT as one way of reducing V T which, in combination with other means of reducing dead space, may be important. One should also consider that reduction of dead space by any means paves the way for using higher RR. Importantly, the clinical implication of this study remains unclear until further studies have been performed in patients of different categories.
This study confirms that changes in pattern of inspiratory gas delivery lead to important instant changes in CO2 elimination and ensuing changes in P aCO2. At square inspiratory flow, these changes relate to MDT in accordance with the hypothesis. At increasing and decreasing inspiratory flow rate, factors other than MDT must be further analysed. Flow rate early in inspiration affects the volume of re-inspired CO2. The effects of changing MDT on CO2 exchange are considerable, particularly at an increased RR. The findings merit further studies in critical care. The trade-off between improved gas exchange related to a prolonged MDT and potential negative effects of higher inspiratory flow rates and/or shorter expiration times should be investigated.
References
Hickling KG, Henderson SJ, Jackson R (1990) Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med 16:372–377
Hickling KG, Walsh J, Henderson S, Jackson R (1994) Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: a prospective study. Crit Care Med 22:1568–1578
Artigas A, Bernard GR, Carlet J, Dreyfuss D, Gattinoni L, Hudson L, Lamy M, Marini JJ, Matthay MA, Pinsky MR, Spragg R, Suter PM (1998) The American–European Consensus Conference on ARDS. Part 2: Ventilatory, pharmacologic, supportive therapy, study design strategies, and issues related to recovery and remodeling. Acute respiratory distress syndrome. Am J Respir Crit Care Med 157:1332–1347
ARDSnetwork (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308
Petrucci N, Iacovelli W (2004) Ventilation with lower tidal volumes versus traditional tidal volumes in adults for acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev CD003844
Kallet RH, Jasmer RM, Pittet JF, Tang JF, Campbell AR, Dicker R, Hemphill C, Luce JM (2005) Clinical implementation of the ARDS network protocol is associated with reduced hospital mortality compared with historical controls. Crit Care Med 33:925–929
Fuleihan SF, Wilson RS, Pontoppidan H (1976) Effect of mechanical ventilation with end-inspiratory pause on blood–gas exchange. Anesth Analg 55:122–130
Dammann JF, McAslan TC, Maffeo CJ (1978) Optimal flow pattern for mechanical ventilation of the lungs. 2. The effect of a sine versus square wave flow pattern with and without an end-inspiratory pause on patients. Crit Care Med 6:293–310
Lachmann B, Jonson B, Lindroth M, Robertson B (1982) Modes of artificial ventilation in severe respiratory distress syndrome. Lung function and morphology in rabbits after wash-out of alveolar surfactant. Crit Care Med 10:724–732
Wolff G, Brunner J, Weibel W, Bowes C (1989) Alveolar efficiency for CO2 elimination and series dead space volume: both are affected by the ventilatory pattern. Appl Cardiopulm Pathol 2:309–314
Mercat A, Diehl JL, Michard F, Anguel N, Teboul JL, Labrousse J, Richard C (2001) Extending inspiratory time in acute respiratory distress syndrome. Crit Care Med 29:40–44
Johansson H, Löfström JB (1975) Effects on breathing mechanics and gas exchange of different inspiratory gas flow patterns during anaesthesia. Acta Anaesthesiol Scand 19:8–18
Al-Saady N, Bennett ED (1985) Decelerating inspiratory flow waveform improves lung mechanics and gas exchange in patients on intermittent positive-pressure ventilation. Intensive Care Med 11:68–75
Markström A, Hedlund A, Lichtwarck-Aschoff M, Nordgren A, Sjöstrand U (2000) Impact of different inspiratory flow patterns on arterial CO2 tension. Ups J Med Sci 105:17–29
Beydon L, Uttman L, Rawal R, Jonson B (2002) Effects of positive end-expiratory pressure on dead space and its partitions in acute lung injury. Intensive Care Med 28:1239–1245
Uttman L, Jonson B (2003) A prolonged postinspiratory pause enhances CO2 elimination by reducing airway dead space. Clin Physiol Funct Imaging 23:252–256
Aboab J, Niklason L, Uttman L, Kouatchet A, Brochard L, Jonson B (2007) CO2 elimination at varying inspiratory pause in acute lung injury. Clin Physiol Funct Imaging 27:2–6
Taskar V, John J, Evander E, Robertson B, Jonson B (1997) Surfactant dysfunction makes lungs vulnerable to repetitive collapse and reexpansion. Am J Respir Crit Care Med 155:313–320
Uttman L, Ögren H, Niklason L, Drefeldt B, Jonson B (2007) Computer simulation allows goal-oriented mechanical ventilation in acute respiratory distress syndrome. Crit Care 11:R36
Svantesson C, Drefeldt B, Sigurdsson S, Larsson A, Brochard L, Jonson B (1999) A single computer-controlled mechanical insufflation allows determination of the pressure–volume relationship of the respiratory system. J Clin Monit Comput 15:9–16
De Robertis E, Liu JM, Blomquist S, Dahm PL, Thorne J, Jonson B (2001) Elastic properties of the lung and the chest wall in young and adult healthy pigs. Eur Respir J 17:703–711
Uttman L, Jonson B (2002) Computer-aided ventilator resetting is feasible on the basis of a physiological profile. Acta Anaesthesiol Scand 46:289–296
Fletcher R, Werner O, Nordström L, Jonson B (1983) Sources of error and their correction in the measurement of carbon dioxide elimination using the Siemens–Elema CO2 Analyzer. Br J Anaesth 55:177–185
De Robertis E, Servillo G, Jonson B, Tufano R (1999) Aspiration of dead space allows normocapnic ventilation at low tidal volumes in man. Intensive Care Med 25:674–679
Weibel ER (1963) Morphometry of the human lung. Springer, Berlin, pp 110–143
Farhi LE, Rahn H (1955) Gas stores of the body and the unsteady state. J Appl Physiol 7:472–484
Taskar V, John J, Larsson A, Wetterberg T, Jonson B (1995) Dynamics of carbon dioxide elimination following ventilator resetting. Chest 108:196–202
Woolcock AJ, Macklem PT (1971) Mechanical factors influencing collateral ventilation in human, dog, and pig lungs. J Appl Physiol 30:99–115
Acknowledgements
This study was supported by the Swedish Research Council (02872) and the Swedish Heart–Lung Foundation. Martina Christensson and Mikael Janiec performed complementary analyses of importance for interpretation of the results.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Åström, E., Uttman, L., Niklason, L. et al. Pattern of inspiratory gas delivery affects CO2 elimination in health and after acute lung injury. Intensive Care Med 34, 377–384 (2008). https://doi.org/10.1007/s00134-007-0840-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-007-0840-7